��Ŀ����
����Ŀ����������A��4�ֳ�����Ԫ����ɣ�Ϊ̽��A����ɣ���Ʋ��������ʵ�飺

��֪��DΪ���ʣ��ڱ�״���µ��ܶ�Ϊ1.25g/L����ҺB�����ԡ�
��1��д�����A�Ľ���Ԫ�������ڱ��е�λ��______________��
��2������D�ĵ���ʽΪ______________��
��3��д��A��H2O2��Ӧ�����ӷ���ʽ______________��
����ijС�����ʵ��̽�����ʵ��¶Ⱥʹ���������SO2��O2��Ӧ��������ijɷ֣�

��1��c��ʢ�ŵ��Լ���______________��e��ʢ�ŵ��Լ���______________��
��2��ijͬѧ����ñ��͵�Na2SO3��Һ����98.3%��ŨH2SO4�������۸÷����Ƿ����______________�������в���˵�����ɣ��������У�����������______________��
���𰸡��������ڵڢ�A�� ![]() 2SCN����11H2O2=2SO42����2CO2����N2����10H2O��2H�� BaCl2��Һ Ʒ����Һ��KMnO4��Һ ������ ��ΪSO3��Na2SO3��Һ��Ӧ����SO2�������ԭ���������SO2�ļ���
2SCN����11H2O2=2SO42����2CO2����N2����10H2O��2H�� BaCl2��Һ Ʒ����Һ��KMnO4��Һ ������ ��ΪSO3��Na2SO3��Һ��Ӧ����SO2�������ԭ���������SO2�ļ���
��������
����DΪ���ʣ��ڱ�״���µ��ܶ�Ϊ1.25g/L����D��Ħ������M=1.25g/L��22.4L��mol��1=28g��mol��1��DΪN2����ҺB�����ԣ����ܹ���Ba(OH)2��Һ���ɰ�ɫ��������BӦ�ú���H2SO4��������ΪH2CO3����ǿ����Һ��H2CO3��ֽ�����CO2����ɫ����Ϊ23.3g����![]() ���������CҲ�ܹ���Ba(OH)2��Һ���ɰ�ɫ������Ӧ����CO2����
���������CҲ�ܹ���Ba(OH)2��Һ���ɰ�ɫ������Ӧ����CO2����![]() ��CO2�����ʵ���Ϊ0.1mol���ڱ���µ����Ϊ2.24L����N2�����Ϊ1.12L�������ʵ���Ϊ0.05mol�����������غ㣬������A����0.1molS��0.1molN��0.1molC��������Ԫ�ص�������Ϊ3.2g��1.4g��1.2g=5.8g����һ��Ԫ������Ϊ9.7g-5.8g=3.9g��S��N��C������Ԫ�ؿ��Թ��ɳ�����������SCN��������һ��Ԫ��ԭ���γɵ������Ӵ�һ������ɣ���Ħ������M=3.9g��0.1mol=39g/mol��ΪKԪ�أ�������ΪKSCN��
��CO2�����ʵ���Ϊ0.1mol���ڱ���µ����Ϊ2.24L����N2�����Ϊ1.12L�������ʵ���Ϊ0.05mol�����������غ㣬������A����0.1molS��0.1molN��0.1molC��������Ԫ�ص�������Ϊ3.2g��1.4g��1.2g=5.8g����һ��Ԫ������Ϊ9.7g-5.8g=3.9g��S��N��C������Ԫ�ؿ��Թ��ɳ�����������SCN��������һ��Ԫ��ԭ���γɵ������Ӵ�һ������ɣ���Ħ������M=3.9g��0.1mol=39g/mol��ΪKԪ�أ�������ΪKSCN��
����SO2��O2�Ʊ�SO3�Ǹ����淴Ӧ����˷�Ӧ��������п϶�����SO2��SO3��O2�������Ҫ�����������SO2��SO3��O2������SO3ʱ����Ҫ��ֹSO2�ĸ��ţ���������BaCl2��Һ��SO3������BaCl2��Ӧ���ɰ�ɫ��������SO2���У�����98.3%H2SO4����δ��Ӧ��SO3����ֹ���SO2�ļ�����ɸ��ţ�SO2�ļ����������Ʒ����Һ��KMnO4��Һ�������ƿ���ռ���O2��
����(1)���A�Ľ���Ԫ��ΪK����Ԫ�����ڱ��е������ڵڢ�A�壻
(2)DΪN2��N��N֮���γ����Թ��õ��Ӷԣ������ʽΪ![]() ��
��
(3)KSCN��H2O2�õ�CO2��N2��SO42���ȣ������ӷ���ʽΪ2SCN����11H2O2=2SO42����2CO2����N2����10H2O��2H����
����SO2��O2�Ʊ�SO3�Ǹ����淴Ӧ����˷�Ӧ��������п϶�����SO2��SO3��O2������SO3ʱ����Ҫ��ֹSO2�ĸ��ţ���������BaCl2��Һ��SO3������BaCl2��Ӧ���ɰ�ɫ��������SO2���У�����98.3%H2SO4����δ��Ӧ��SO3����ֹ���SO2�ļ�����ɸ��ţ�SO2�ļ����������Ʒ����Һ��KMnO4��Һ�������ƿ���ռ���O2��
(1)c��ʢ�ŵ��Լ���BaCl2��Һ��e��ʢ�ŵ��Լ�ΪƷ����Һ��KMnO4��Һ��
(2)��������Na2SO3����98.3%��ŨH2SO4����ΪSO3��Na2SO3��Һ��Ӧ����SO2�������ԭ���������SO2�ļ��顣

����Ŀ����ϩ(C3H6)����Ҫ���л�����ԭ�ϡ�����ֱ�������Ʊ�ϩ�����ķ�Ӧ�У�
��Ӧ����C3H8(g)C3H6(g)+H2(g) ��H1
��Ӧ����2C3H8(g)3C2H4(g)+2H2(g) ��H2
��֪������Ӧ�����Ea(��)С�ڷ�Ӧ�����Ea(��)��
��3�����ʵ�ȼ�������±���
C3H8(g) | C3H6(g) | H2(g) |
��2217.8kJ��mol-1 | ��2058kJ��mol-1 | ��285.8kJ��mol-1 |
��1����H1=_______kJ��mol-1
��2�����º��������£����ܱ������г���1molC3H8(g)�����������˵�����������Ʊ�ϩ��Ӧ�ﵽƽ��״̬����______________��
A���÷�Ӧ��H���ֲ���B����������ƽ��Ħ���������ֲ���
C��![]() ���ֲ���D��C3H8�ֽ�������C3H6�����������
���ֲ���D��C3H8�ֽ�������C3H6�����������
��3��һ���¶��£�������ܱ������г��������ʵ���Ϊ4mol�ı��������������壨�����������ǻ����������Ӧ��ͬʱ�䣬��ϩ������������Ĺ�ϵ��ͼ1��ʾ��ͼ���������ߵ�ԭ����______________��
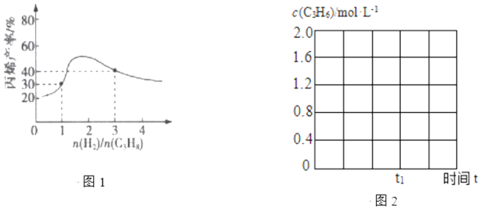
��4������Ӧ�¶�600������10molC3H8(g)����2L�ĸ��������У�����ƽ��ת�������ϩ��ѡ���Ծ�Ϊ40%����Ӧ����ƽ�ⳣ��K=______________��������2λС��������ϩ��ѡ����=![]() ��100%��
��100%��
��600�����������ⷴӦ��t1ʱ��ƽ�⣬����ͼ2�л�����������c(C3H6)��ʱ��t�ı仯ͼ��______________
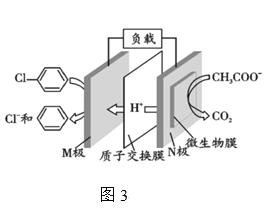
��5����һ�ȱ���(C3H7Cl)�ķ�ˮ��ͨ���������������ƣ���Ƴ������س�ȥ����ԭ����ͼ3��ʾ��д��N�缫�ĵ缫��Ӧʽ______________��