��Ŀ����
A��B��C��D��E��������ѧ�����ĵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ���塣E�ǵؿ��к����ӵڶ�λ�Ľ�����D�ɷֱ��A��B��C��һ���������»��ϣ�����X��Y��Z��Y��Z��Ne�ĵ�������ȣ�A�ǵ������ڵ�Ԫ�ء��йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ����
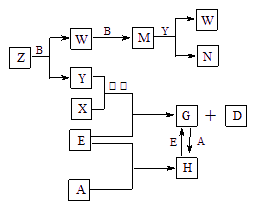
��1��A�Ļ�ѧʽΪ ��B�Ļ�ѧʽΪ ��C�ĵ���ʽΪ ��
��2��Z��W�ڴ����ͼ��ȵ������·�Ӧ����C��Y������һ����������ķ�Ӧ����������W�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3����N��Һ����G����Һ�л����W��д�������仯�����ӷ�Ӧ����ʽ��
��
��4��Z��Nǡ�÷�Ӧ������ܽ���ˮ�У�������Һ��pH������ڡ�����С�ڡ����ڡ��� 7�������ӷ�Ӧ����ʽ��ʾ��ԭ��Ϊ ��
��14�֣���1��Cl2��2�֣� O2��2�֣� ![]() ��2�֣�
��2�֣�
��2��6NO��4NH3![]() 5N2��6H2O��2�֣� ��3��4H+��NO3�C��3Fe2+
5N2��6H2O��2�֣� ��3��4H+��NO3�C��3Fe2+![]() NO����2H2O��3Fe3+��2�֣�
NO����2H2O��3Fe3+��2�֣�
��4��С��7��2�֣� NH4+��H2O![]() NH3��H2O��H+��2�֣�
NH3��H2O��H+��2�֣�
����:
��

| A�������ӵİ뾶��C��D��E��B | B����ҵ�ϳ��õ�ⷨ�Ƶ�C��D�ĵ��� | C���ȶ��ԣ�A2B��A2E | D������D������ұ��ijЩ���۽��� |

 2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
