��Ŀ����
λ�ڶ����ڵ���������Ԫ��A��B��C��Dԭ����������������֪A��Cλ��ͬһ���壬A�����ڱ���ԭ�Ӱ뾶��С��B��D��������������ȣ���B��D��ԭ������֮��ΪA��Cԭ������֮�͵���������������ƶ�����������⣺
(1)д��������Ԫ���γɵľ���Ư�����õ��������ʵĻ�ѧʽ___________��___________��___________��___________��
(2)C��D�γ�D����ͼۻ�����ĵ���ʽ��______________________��
(3)����B��C��D����Ԫ���γɵ�һ���Σ�����ˮ��ʼ��ԣ�����һ�����ӷ���ʽ��ʾ��ʼ��Ե�ԭ��____________________________________________��
(1)д��������Ԫ���γɵľ���Ư�����õ��������ʵĻ�ѧʽ___________��___________��___________��___________��
(2)C��D�γ�D����ͼۻ�����ĵ���ʽ��______________________��
(3)����B��C��D����Ԫ���γɵ�һ���Σ�����ˮ��ʼ��ԣ�����һ�����ӷ���ʽ��ʾ��ʼ��Ե�ԭ��____________________________________________��
(1)SO2 O3 H2O2 Na2O2
(2)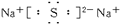
(3)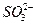 +H2O
+H2O
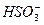 +OH-
+OH-
(2)
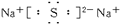
(3)
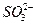 +H2O
+H2O
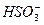 +OH-
+OH-������Ԫ�ػ�������ƶ�Ϊ���壬��������Һ�����������ˮ��Ĺ�ϵ�Լ�����ˮ�ⷽ��ʽ����д���������֪��AΪHԪ�أ�BΪOԪ�أ�CΪNaԪ�أ�DΪSԪ�ء������ƶϳ�������Ԫ���γɵľ���Ư�����õ��������ʵĻ�ѧʽ����B��C��D����Ԫ���γɵ�ˮ��Һ�ʼ��Ե���ΪNa2SO3��ԭ����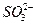 ����ˮ�⡣
����ˮ�⡣
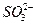 ����ˮ�⡣
����ˮ�⡣
��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
 )+c(
)+c( )+c(OH-)
)+c(OH-) ��CO2����ȵ�����
��CO2����ȵ����� )�ӽ�0.1 mol��L-1��Ӧ��ȡ( )
)�ӽ�0.1 mol��L-1��Ӧ��ȡ( ) ��Һ�У�
��Һ�У�

 ��
�� ��Һ�У�
��Һ�У�
 ��������
�������� ��
��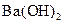 ��Һ�������Ϻ���Һ��һ����
��Һ�������Ϻ���Һ��һ����
 ��ˮ�м�������
��ˮ�м������� ���壬��Һ��
���壬��Һ�� ����
����