��Ŀ����
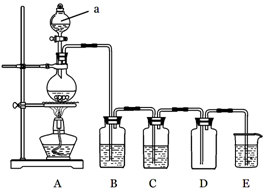
����14�֣���18mol/L��Ũ��������3mol/L��ϡ����100mL������ȷ�IJ���˳�����
���������ڲ���д���пո�
�� )����������ˮϴ��С�ձ�����ϴ��Һ��_______ע��__________���ظ�2�Ρ�
�� )��Ũ�������ձ�������ע��ʢ����������ˮ��С�ձ��У����ò��������Ͻ��衣
�� )����ͲȡŨ����______mL��
�� )������ƿ�ǽ�ҡ�ȡ�
�� )������ȴ��������Һ��________ ע��________ �С�
�� )���ý�ͷ�ιܼ�����ˮ��ʹ��Һ��Һ����ʹ�ǡ����̶������С�
�� )����������ƿ��С�ļ�����ˮ��ֱ���ӽ��̶���__________cm����
��ʵ�����������������������Һ����������ʵ���Ũ���к�Ӱ�죨��ƫ�ߡ�
ƫ�͡���Ӱ��)��
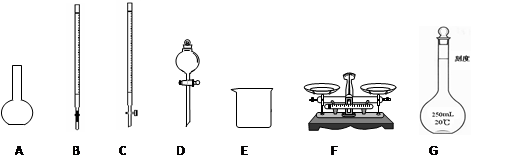
��1) ����Ͳ��ȡŨ����ʱ���ӿ̶���___________��
��2) ϡ�����в���������Һ�ν���____________��
��3) δ��ϴ��Һ��������ƿ___________��
��4) ����ʱ���ӿ̶���__________��
��5) ҡ�Ⱥ��ְ�Һ���½����ּ�����ˮ���̶���__________��
��6) ����ǰ����ƿ��ˮϴ�Ӻ�δ���и��ﴦ��__________��
���������ڲ���д���пո�
�� )����������ˮϴ��С�ձ�����ϴ��Һ��_______ע��__________���ظ�2�Ρ�
�� )��Ũ�������ձ�������ע��ʢ����������ˮ��С�ձ��У����ò��������Ͻ��衣
�� )����ͲȡŨ����______mL��
�� )������ƿ�ǽ�ҡ�ȡ�
�� )������ȴ��������Һ��________ ע��________ �С�
�� )���ý�ͷ�ιܼ�����ˮ��ʹ��Һ��Һ����ʹ�ǡ����̶������С�
�� )����������ƿ��С�ļ�����ˮ��ֱ���ӽ��̶���__________cm����
��ʵ�����������������������Һ����������ʵ���Ũ���к�Ӱ�죨��ƫ�ߡ�
ƫ�͡���Ӱ��)��
��1) ����Ͳ��ȡŨ����ʱ���ӿ̶���___________��
��2) ϡ�����в���������Һ�ν���____________��
��3) δ��ϴ��Һ��������ƿ___________��
��4) ����ʱ���ӿ̶���__________��
��5) ҡ�Ⱥ��ְ�Һ���½����ּ�����ˮ���̶���__________��
��6) ����ǰ����ƿ��ˮϴ�Ӻ�δ���и��ﴦ��__________��
������˳��2�֣�����ÿ��1�֣�
(4) ������ 100ml����ƿ
��2��
(1) 16.7ml
��7��
��3�������� 100ml����ƿ
��6��
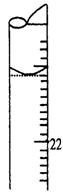
��5��1��2cm
��1������5��ƫ�ͣ���6����Ӱ��
(4) ������ 100ml����ƿ
��2��
(1) 16.7ml
��7��
��3�������� 100ml����ƿ
��6��
��5��1��2cm
��1������5��ƫ�ͣ���6����Ӱ��
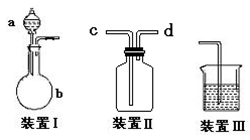
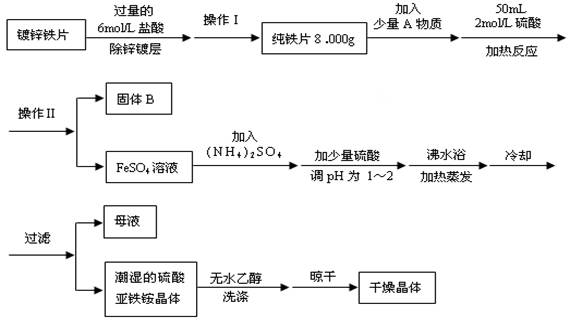
1��ʵ�鲽�輰������
�������������ȡ�����ܽ⣨ϡ�ͣ�����ȴ��ת�ơ�ϴ�ӡ������ݡ�ҡ�ȡ�װƿ��ǩ
2��ע������
�� ����������Һ�����ѡȡ��Ӧ��������ƿ������950mLijŨ����ҺӦѡ1000mL������ƿ,ȷ������ʱ,���ܰ���950mL����,Ҫ����1000mL���㡣
�� ����ƿ��ʹ��ǰҪ����Ƿ�©ˮ��
������a����ˮ���������۲��������
b��ƿ����ת1800���������۲졣
����鲻©ˮ������ƿ����ʹ�ã�ʹ��ǰ���������ƿϴ�Ӹɾ��������ظ��
�� ����ƿ�в��ܽ������Ũ��Һֱ���ܽ��ϡ��,������Ϊ��Ӧ����,Ҳ������������������Һ��
�� ��Һע������ƿǰ��ָ������¡�������ƿ��ת����Һ���������ˮʱ����Ҫ�ò�����������
�� ������ƿ��Һ��ռ�ݻ�2/3����ʱӦ����ҡ�ȡ�
�� ������ƿ��ʹ�ù�����,�ƶ�����ƿ,��Ӧ����ƿ���̶������ϲ�λ,����ƿ����Һ���ȶ���������仯,ʹ��ҺŨ�Ȳ�ȷ��
�� �ڶ�ȡ����ƿ��Һ�����ʱ��Ҫʹ�۾�������������ƿ�Ŀ̶���ƽ�С���Һ�尼Һ��������ƿ�Ŀ̶���ǡ������ʱ������ֹͣ�μ�����ˮ��
�� ʵ�������ʱϴ������ƿ��
3.����c��
 ��
�� �� �ж�������Һ����������n��V��m��V����
�� �ж�������Һ����������n��V��m��V����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Cl2����MnCl2��2H2O��
Cl2����MnCl2��2H2O��