��Ŀ����
13������˵����ȷ���ǣ��������ٵ��ۡ���ά�ء���֬�������ʶ�����Ȼ�л��߷��ӻ�����
�ڵ��ۡ���ά�ء����Ƕ��Ƿǻ�ԭ����
������Ӫ�������ࡢ��֬���������У���֬��������ߵ�Ӫ����
���ô��Na2CO3������Һϴ�Ӳ;��ϵ�����
����ˮʹ��īˮ��ɫԭ����CH2=CH2ʹBr2��CCl4��Һ��ɫԭ����ͬ
���������ȼ����Ҫ��ѹ����Ȼ����Һ��ʯ�������࣬���Ǿ�����̼�⻯���
| A�� | �٢ڢ� | B�� | �ڢۢܢ� | C�� | �ڢܢݢ� | D�� | �ۢܢ� |
���� ����֬����Է���������С����������Ȼ�л��߷��ӻ����
�ڵ��ۡ���ά�ء����Ƕ�����ȩ�������Ƿǻ�ԭ���ǣ�
�۵��ۡ�����͵������У���֬��������ߵ�Ӫ���أ�
�ܴ���Ϊ̼������Һ��̼������Һ�ʼ��ԣ��ܹ��ܽ����ۣ�
����ˮʹ��īˮ��ɫ���õ���ǿ�����ԣ���CH2=CH2ʹBr2��CCl4��Һ��ɫ�Ƿ����˼ӳɷ�Ӧ��
��ѹ����Ȼ������Ҫ�ɷ�Ϊ���飬Һ��ʯ����Ϊ����Ļ���
��� �⣺�ٵ��ۡ������ʺ���ά��Ϊ��Ȼ�߷��ӻ��������֬����Է���������С���������л��߷��ӻ�����ʢٴ���
�ڵ��ۡ���ά�ء����Ƿ����ж�����ȩ�����������Ƕ��Ƿǻ�ԭ���ǣ��ʢ���ȷ��
������Ӫ�������У��������࣬����֬��������ߵ�Ӫ���أ��ʢ���ȷ��
��̼������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���֬�ܹ��ڼ�����Һ�з���ˮ�ⷴӦ�������ô��Na2CO3��������Һ���Գ�ȥ���ۣ��ʢ���ȷ��
�������еĴ��������ǿ�����ԣ��ܹ�ʹ��īˮ��ɫ������CH2=CH2ʹBr2��CCl4��Һ��ɫ�Ƿ����˼ӳɷ�Ӧ�����ߵ���ɫԭ����ͬ���ʢݴ���
��ѹ����Ȼ������Ҫ�ɷ�Ϊ���飬Һ��ʯ����Ϊ����Ļ������Ƕ���̼�⻯����ʢ���ȷ��
��ѡB��
���� ���⿼��߷��ӻ�������жϡ�������Ư���ԡ���֬����������ʡ�����Ӫ�����ʣ���Ŀ�ѶȲ���ע�����ճ����л���ṹ�����ʣ���ȷ������Ư��ԭ��������֪ʶ��϶࣬��ֿ�����ѧ�����Ӧ�û���֪ʶ��������

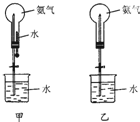 ��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ��
��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����1��д��ʵ������ȡ�����Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2���ռ�����ʹ�������ſ���������Ҫ�õ�����İ�����ѡ�ü�ʯ�����������
��3���ü�װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ����Ǵ�ֹˮ�У�������ͷ�ι��е�ˮ����ʵ���ԭ���ǰ��������ܽ���ˮ����ʹ��ƿ������ѹǿѸ�ټ�С��
��4�����ֻ�ṩ��ͼװ�ã������������Ȫ�ķ������ӣ����֣�����ë���ȣ�����ƿ���ȣ����������������ͣ��ϳ����������ڵĿ�����������ˮ�Ӵ�����������Ȫ��
��5���ֽ����е�NH3��ˮ�������������Һ�壬����ȫ����������װ���е�ѡ���ǣ���˵�����ɣ��ɲ���������
| ���� | Һ�� | |
| A | NO2 | ˮ |
| B | CO2 | 4mol•L-1NaOH��Һ |
| C | Cl2 | ����NaCl��Һ |
| D | NH3 | 1mol•L-1���� |
��ѡ����C�����������������ڱ����Ȼ�����Һ��
��ѡ���ǣ��������ǣ���
��ѡ���ǣ��������ǣ���
| A�� | ��ͨ��������Ҫ�ɷ��Ǵ��ʯ��ʯ�Ͷ������� | |
| B�� | ���⻯ѧ��������̼�⻯����͵���������Ĵ����ŷ��й� | |
| C�� | ������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ | |
| D�� | C��S�ֱ��ڿ�����ȼ�վ��ɵõ����ֲ�ͬ�������� |
| A�� | ����CuSO4��Һ��Ӧ��3Al+2Cu2+�T2Cu+3Al3+ | |
| B�� | ��Ba��OH��2��Һ�мӹ���NaHCO3��Һ��HCO3-+Ba2++OH-�TH2O+BaCO3�� | |
| C�� | ����ʯ��ˮ�����ᷴӦ��H++OH-�TH2O | |
| D�� | �Ȼ�����Һ�м��������ˮ��Al3++4NH3•H2O�T4NH4++AlO2-+2H2O |
| A�� | �ý����Ƽ���ƾ����Ƿ���ˮ | |
| B�� | Ϊ��ȥ���е��������ӣ��������м�����������ˮ����ˣ���ȥ���� | |
| C�� | Ϊ��ȥ�����е�������Ȳ�����������ͨ�����Ը��������Һ���� | |
| D�� | ��C2H5Br��NaOH��Һ��Ϻ�����ϡ�����ữ���ټ���AgNO3��Һ������C2H5Br�е���Ԫ�� |
 ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ��������ģ����ͼ��ʾ�������й�������ȷ���ǣ�������
ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ��������ģ����ͼ��ʾ�������й�������ȷ���ǣ������� �ٷ���ʽΪC10H9O3��
�ڲ�������ˮ��
��1mol�������������4molH2�ӳɡ�
����ʹ����KMnO4��Һ��ɫ��
��1mol������������뺬2mol NaOH����Һ��Ӧ��
| A�� | �٢ڢ� | B�� | �ۢܢ� | C�� | �ڢۢ� | D�� | �ڢۢܢ� |
| A�� | ��BaCl2��Һ��ͨ��SO2���壬���а�ɫ�������������ʧ | |
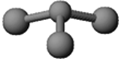
| B�� | CCl4��NH3�������и�ԭ������������8���ӽṹ | |
| C�� | ��������Ԫ��R2+��M+�ĵ��Ӳ�ṹ��ͬ���������R��M | |
| D�� | ��״���£�11.2 LN2��O2�Ļ��������ԭ������ԼΪ6.02��1023 |
 A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㣬DԪ�ص�ԭ������������Ϊm������������Ϊn��EԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊ$\frac{m}{2}$-n����ش��������⣺
A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㣬DԪ�ص�ԭ������������Ϊm������������Ϊn��EԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊ$\frac{m}{2}$-n����ش��������⣺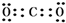 ��
��