��Ŀ����
����Ŀ�� Kx[Cu(C2O4)y]��zH2O (ˮ�ϲ���ͭ(��)���)��һ�ֻ���ԭ�ϣ�ʵ�����Ʊ�����ˮ�ϲ���ͭ��ز��ⶨ��Ʒ����ɣ�ʵ�鲽�����£�
I�Ʊ�CuO
��ͼ1��ʾװ�ý���Һ��Ϻ�С���������ɫ�������ɫ����CuO�����5~10���ӡ�����ȴ��ȫ��ת����ͼ2װ�ù��ˣ���������ˮϴ�ӳ���2~3�Ρ�
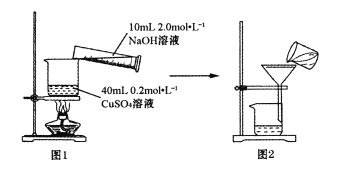
(1)��ָ��ͼ2װ���в����еĴ���___________��ͼ2������������ˮϴ�ӳ���2~3�Σ���ĿΪ___________��
�ڼ������ϴ�Ӹɾ��ķ���Ϊ___________��
���Ʊ�KHC2O4��K2C2O4�����Һ
��ȡ3.0g H2C2O4��2H2O����250mL�ձ��У�����40mL����ˮ����(�¶ȵ���80��)�ܽ⡣���������μ���2.2g��ˮK2CO3����ַ�Ӧ�����ɺ�KHC2O4��K2C2O4�Ļ���
(2)�ò��������û������n(KHC2O4)��n(K2C2O4)=____________��(ȡ����)
���Ʊ�ˮ�ϲ���ͭ��ؾ���
��KHC2O4��K2C2O4�����Һˮԡ�ȣ��ٽ�CuO��ͬ��ֽһ����뵽����Һ�У���ַ�Ӧ��CuO����ȫ���ܽ⣬ȡ����ֽ����Ũ������ȴ�ᾧ�����ˣ����Ҵ���ϴ����Ȼ���ɣ������õ���Ʒ2.9760g(������ƽ����)��
(3)�ٲ�������KHC2O4��������__________��
�����Ҵ���ϴ������ˮ���ŵ���__________��
���ܽ�CuO����ʱ����ͬ��ֽһ����˵���Һ�е�Ŀ����__________��
���ⶨˮ�ϲ���ͭ��ؾ���ijɷ�
ȡ�����Ƶõ���Ʒ�����Һ���ñ������{�������Һ�ζ�![]() ���ñ�EDTA��Һ(PARΪָʾ��)�ζ�Cu2+����������Ʒ��n(Cu)��n(
���ñ�EDTA��Һ(PARΪָʾ��)�ζ�Cu2+����������Ʒ��n(Cu)��n(![]() )=1:2�������Ʊ���Ʒ�Ĺ�����Cu2+����ġ�
)=1:2�������Ʊ���Ʒ�Ĺ�����Cu2+����ġ�
(4)ˮ�ϲ���ͭ(��)��ؾ���Ļ�ѧʽΪ__________��
���𰸡�����ʱת�ƹ�Һ�����û��ͨ������������ ��ȥ�������������Ŀ������������� ȡ�������һ��ϴ������Һ���Թ��У�����BaCl2��Һ�����ް�ɫ�������ɣ����Ѿ�ϴ�Ӹɾ� 2:1 �ӿ�CuO���ܽ�(�����Ի����ٽ�CuO���ܽ�) ����ˮ�ϲ���ͭ��ص��ܽ���ģ��ӿ쾧�����ˮ�ֻӷ� ��ֹCuO����� K2[Cu(C2O4)2]��3H2O
��������
��ʵ���Ŀ�����Ʊ�����ˮ�ϲ���ͭ��ز��ⶨ��Ʒ����ɣ�������������ͭ������������Һ��ȡ������ͭ����������ʹ��ֽ�õ�CuO��Ȼ��H2C2O4��2H2O����ˮK2CO3�Ʊ�KHC2O4��K2C2O4�����Һ��Ȼ��KHC2O4��K2C2O4�����Һˮԡ�ȣ��ٽ�CuO��ͬ��ֽһ����뵽����Һ�У���ַ�Ӧ����ϵ�в����õ���Ʒ�����ⶨˮ�ϲ���ͭ��ؾ���ijɷ֡�
(1)��ͼ2װ���в����еĴ���Ϊ������ʱת�ƹ�Һ�����û��ͨ��������������Ϊ��ȥ�������������Ŀ������������ӣ���Ҫ������ˮϴ�ӳ���2~3�Σ���
�ڵõ���CuO���������ܸ����������ƣ�����ͨ�������������ȷ�������Ƿ�ϴ�Ӹɾ������巽��Ϊ��ȡ�������һ��ϴ������Һ���Թ��У�����BaCl2��Һ�����ް�ɫ�������ɣ����Ѿ�ϴ�Ӹɾ���
(2)3.0g H2C2O4��2H2O�����ʵ���Ϊ![]() ��0.0238mol��2.2g��ˮK2CO3�����ʵ���Ϊ
��0.0238mol��2.2g��ˮK2CO3�����ʵ���Ϊ![]() ��0.0159mol�������̼Ԫ���غ�ɵã�n(KHC2O4)+n(K2C2O4)=0.0238 mol������KԪ���غ�ɵ�n(KHC2O4)+2n(K2C2O4)=(0.0159��2)mol���������n(KHC2O4)=0.0158mol��n(K2C2O4)=0.0080mol������n(KHC2O4)��n(K2C2O4)=2:1��
��0.0159mol�������̼Ԫ���غ�ɵã�n(KHC2O4)+n(K2C2O4)=0.0238 mol������KԪ���غ�ɵ�n(KHC2O4)+2n(K2C2O4)=(0.0159��2)mol���������n(KHC2O4)=0.0158mol��n(K2C2O4)=0.0080mol������n(KHC2O4)��n(K2C2O4)=2:1��
(3)��KHC2O4���Ե���������ӣ��ӿ�CuO���ܽ⣻
�����Ҵ���ϴ���Լ���ˮ�ϲ���ͭ��ص��ܽ���ģ�ͬʱ�Ҵ��ӷ����Լӿ쾧�����ˮ�ֻӷ���
����ֽ�ϸ�����CuO����ͬ��ֽһ����˵���Һ�п��Լ���CuO����ģ�
(4)��֪��Ʒ��n(Cu)��n(![]() )=1:2������y=2�����ݵ���غ��֪x=2�����Ը���n(K2C2O4)=0.0080mol����֪n(K2[Cu(C2O4)2]��zH2O)=0.0080mol����Ʒ����Ϊ2.9760g��������
)=1:2������y=2�����ݵ���غ��֪x=2�����Ը���n(K2C2O4)=0.0080mol����֪n(K2[Cu(C2O4)2]��zH2O)=0.0080mol����Ʒ����Ϊ2.9760g��������![]() =0.0159mol�����z=3������ˮ�ϲ���ͭ(��)��ؾ���Ļ�ѧʽΪK2[Cu(C2O4)2]��3H2O��
=0.0159mol�����z=3������ˮ�ϲ���ͭ(��)��ؾ���Ļ�ѧʽΪK2[Cu(C2O4)2]��3H2O��

����Ŀ������ʵ�鷽�����ܴﵽԤ��Ŀ�ĵ���
ʵ�鷽�� | ʵ��Ŀ�� | |
A | ����֧�Թ��зֱ���� | ̽�� |
B | ȡ | ����8%�� |
C | �� | �Ƚ� |
D | ��ͬ��ͬŨ�� | �Ƚ������Ԫ�طǽ�����ǿ�� |
A.AB.BC.CD.D