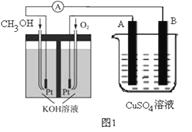
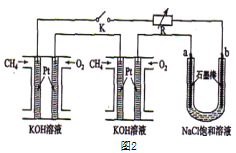
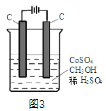
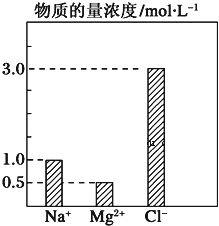
��Ŀ����
����Ŀ��A��B��C��D��E��F�����ֶ����ڵ�����Ԫ�أ�ԭ��������������A��IA��ķǽ���Ԫ�أ�BԪ�ص�����������ˮ�����������̬�⻯��ɷ�Ӧ�����Σ�C�Ƕ�������ԭ�Ӱ뾶����Ԫ�أ�DԪ��ԭ��L���������M�������֮�����BԪ��������������EԪ��������ۺ�����۴����͵���4���ݴ˻ش��������⣺
��1��FԪ�������ڱ��е�λ��__________��Cԭ�ӽṹʾ��ͼ______________��
��2��A��B��Ԫ�ؿ��γ�18���ӵķ��ӣ��÷��ӵĵ���ʽΪ_______________��
��3��A����Ԫ���γɵļ�������۷е����A��E�γɵļ����ԭ����____________��
��4��C��D��F���γɸ��ӻ�����C[DF4]���û����ﺬ�еĻ�ѧ������Ϊ________________��
��5����������Ԫ���γɵĻ�������ʼ��ijЩ��Ӧ��������˵��E��F��Ԫ�طǽ����Ե�ǿ����д������һ�����ӷ�Ӧ����ʽ__________________________________��
��6��д��C��D��Ԫ������������ˮ���ﷴӦ�����ӷ���ʽ____________________��
���𰸡� ��3����VIA 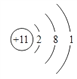
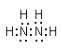 ˮ���Ӽ���γ�����������۷е����H2S ���ۼ������Ӽ� H2S + Cl2 = 2H+ + Cl- + S �� S2- + Cl2 = S + 2Cl- Al(OH)3 + OH- = [Al(OH)4]-
ˮ���Ӽ���γ�����������۷е����H2S ���ۼ������Ӽ� H2S + Cl2 = 2H+ + Cl- + S �� S2- + Cl2 = S + 2Cl- Al(OH)3 + OH- = [Al(OH)4]-
��������A��IA��ķǽ���Ԫ�أ�IA���Ԫ����ֻ��H�Ƿǽ���Ԫ�أ�����A��HԪ����BԪ�ص�����������ˮ�����������̬�⻯��ɷ�Ӧ�����Σ�ֻ��NԪ�ط���Ҫ������B��NԪ������������ԭ�Ӱ뾶����Ԫ����NaԪ��������C��NaԪ�أ�BԪ����NԪ����������������5����DԪ��ԭ��L���������M�������֮�����BԪ������������������DԪ����AlԪ�أ�EԪ��������ۺ�����۴����͵���4��˵��EԪ���������6�����ӣ�����E��ԭ����������D������E��SԪ�أ�F��ԭ����������E����F������Ԫ�أ�����Fֻ����ClԪ�ء�
��1��F��ClԪ��,�����ڱ��е�λ���ǵ�3����VIA ��C��NaԪ�أ�ԭ�ӽṹʾ��ͼΪ�� ����Ϊ����3����VIA
��������3����VIA 
��2��H��NԪ�ؿ����γ�18���ӵķ��ӣ��÷���ΪN2H4�������ʽΪ��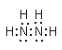 ����Ϊ��
������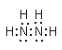
��3��A����Ԫ���γɵļ�����Ϊ:H2O,A��E�γɵļ�����ΪH2S��H2O�������γ��������H2S���Ӳ����γ�����������۷е����H2S����Ϊ��ˮ���Ӽ���γ�����������۷е����H2S
(4)C��D��F�γɵĸ��ӻ�����C[DF4]ΪNa[AlCl4]�����й��ۼ������Ӽ��� ��Ϊ�����ۼ������Ӽ�
��5�������ܰ���������Ϊ��������ʽΪ��H2S + Cl2 = 2H+ + Cl- + S �� S2- + Cl2 = S + 2Cl- ����Ϊ��H2S + Cl2 = 2H+ + Cl- + S �� S2- + Cl2 = S + 2Cl-
��6��C��D��Ԫ������������ˮ����ֱ�Ϊ:NaOH��Al(OH)3�����߷�Ӧ�����ӷ���ʽΪ��Al(OH)3 + OH- = [Al(OH)4]-����Ϊ��Al(OH)3 + OH- = [Al(OH)4]-

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�