��Ŀ����
��֪���з�Ӧ���Ȼ�ѧ����ʽ��
6C(s)��5H2(g)��3N2(g)��9O2(g)=2C3H5(ONO2)3(l) ��H1
2H2(g)��O2(g)=2H2O(g) ��H2
C(s)��O2(g)=CO2(g) ��H3
��Ӧ4C3H5(ONO2)3(l)=12CO2(g)��10H2O(g)��O2(g)��6N2(g)�Ħ�HΪ(����)��
| A��12��H3��5��H2��2��H1 | B��2��H1��5��H2��12��H3 |
| C��12��H3��5��H2��2��H1 | D����H1��5��H2��12��H3 |
��A
����

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д����������ɷ������·�Ӧ��P4+5O2=P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��P akJ��mol��1��P��O bkJ��mol��1��P="O" ckJ��mol��1��O=O dkJ��mol��1��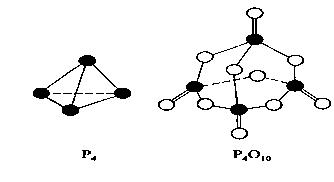
����ͼʾ�ķ��ӽṹ���й����ݹ���÷�Ӧ�ġ�H��������ȷ����
| A����4c+12b��6a��5d��kJ��mol��1 |
| B����6a+5d��4c��12b��kJ��mol��1 |
| C����4c+12b��4a��5d��kJ��mol��1 |
| D����4a+5d��4c��12b��kJ��mol��1 |
����˵����ȷ����
| A����Ȼ����ʯ�Ͷ������Ŀ�������Դ |
| B����ȼú������̼��ƻ���ʯ�ҿ��Լ���SO2���ŷ� |
| C����AgC1������Һ�м���AgNO3��Һ����ƽ��ʱ����Һ��Ksp(AgCl)���� |
| D���ع��ͺͿ����Ͷ������������� |
���з�Ӧ�е������仯��ϵ������ͼ��ʾ����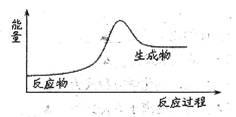
| A���������ռӦ | B����Ȼ��ȼ�� |
| C������������ˮ��Ӧ | D������ʯ��ʯ |
FeCl3(aq)��KSCN(aq)���ʱ��������ƽ��:Fe3+(aq)+SCN-(aq) Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c��Fe(SCN)2+�����¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����( )
Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c��Fe(SCN)2+�����¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����( )
| A��FeCl3(aq)��KSCN(aq)��Ӧ���Ȼ�ѧ����ʽΪ:Fe3+(aq)+SCN-(aq)=Fe(SCN)2+(aq) ��H��0 |
| B���¶�ΪT1��T2ʱ����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2 |
| C����Ӧ����D��ʱ��һ����v(��)��v(��) |
| D��A����B����ȣ�A���c(Fe3+)�� |
��֪1 mol����ת��Ϊ1 mol���ף�����18.39 kJ������
��4P(�죬s)��5O2(g)=2P2O5(s)����H1
��P4(�ף�s)��5O2(g)=2P2O5(s)����H2
��H1�릤H2�Ĺ�ϵ��ȷ����(����)
| A����H1����H2������������ | B����H1>��H2 |
| C����H1<��H2 | D����ȷ�� |
��֪�����Ȼ�ѧ����ʽ��
��H2��g��+1/2O2��g��=H2O��g�� ��H1��a kJ��mol��1
��2H2��g��+O2��g��=2H2O��g�� ��H2��b kJ��mol��1
��H2��g��+1/2O2��g��=H2O��l�� ��H3��c kJ��mol��1
��2H2��g��+O2��g��=2H2O��l�� ��H4��d kJ��mol��1
���й�ϵʽ����ȷ���ǣ� ��
| A��a��c��0 | B��b��d��0 |
| C��2a��b��0 | D��2c��d��0 |
����˵�����ʾ������ȷ���� (����)��
| A����Ӧ��������������������������ʱ����Ӧһ�������Է����� |
| B����֪��H2S(g)��aO2(g)=x��bH2O(l)����H������H��ʾH2S��ȼ���ȣ���xΪSO2(g) |
| C����֪��2SO2(g)��O2(g)??2SO3(g)����H����98.3 kJ��mol��1�����ܱ������г���1 mol SO2��0.5 mol O2����ַ�Ӧ��ų�49.15 kJ������ |
| D����ʯī�Ƚ��ʯ�ȶ��ɵã�C(s�����ʯ)=C(s��ʯī)����H>0 |
��100 g̿����ȫȼ������������CO��CO2�������Ϊ1��2����֪��
C��s����1/2O2��g��=CO��g�� ��H1����110��35 kJ/mol
CO��g����1/2O2 =CO2��g�� ��H2����282��57 kJ/mol
����100 g̿��ȫȼ����ȣ���ʧ�������ǣ� ��
| A��392��93 Kj | B��2 489��42 kJ |
| C��784��92 kJ | D��3 274��3 kJ |