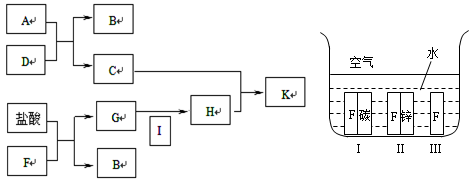
��Ŀ����
��֪ͨ��״���¼ס��ҡ���������Ϊ���嵥�ʣ�A��B��C��D��E��F��G��H��Ϊ���������A��B��E��G��Ϊ����,CΪ����Һ�塣��Ӧ�١��ڡ��۶�����Ҫ�Ļ�����Ӧ����Ӧ������Ҫ��ʵ������ȡ����ķ�Ӧ���йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����

��ش��������⣺
(1)��Ӧ�ܵĻ�ѧ����ʽΪ: ________________________��
(2)B��E��һ�������¿ɷ�����Ӧ,����һ������ʵ������ķ�Ӧ,������E�Ի�������Ⱦ���÷�Ӧ���������뻹ԭ��������ʵ���֮��Ϊ________��
(3)0.1mol lL-1A��Һ��0.1mol ?L-1B��Һ�������ϣ���Һ��________��,ԭ����(�����ӷ���ʽ˵��) ________________��
(4)�����ʵ�����D�����е�������(����ʵ�����������ͽ��ۣ���________________��
(5)pH��ͬ��A��D��H������Һ����ˮ�������c(OH��)�Ĵ�С��ϵ��(��A��D��H��ʾ) : ____________��
(6)��һ������Fe��FeO��Fe3O4�Ļ�����У�����1mol? L��1 A����Һ100 mL��ǡ��ʹ�����ȫ���ܽ⣬�ҷų�336mL(��״����)�����壬��������Һ�м���KSCN��Һ����Һ��ɫ���֣���ȡͬ������Fe��FeO��Fe3O4��������1 mol ? L��1 H����Һ,Ҳǡ��ʹ�����ȫ���ܽ⣬����������Һ�м���KSCN��Һ,��ҺҲ��ɫ����,���������H��Һ�������________��

��ش��������⣺
(1)��Ӧ�ܵĻ�ѧ����ʽΪ: ________________________��
(2)B��E��һ�������¿ɷ�����Ӧ,����һ������ʵ������ķ�Ӧ,������E�Ի�������Ⱦ���÷�Ӧ���������뻹ԭ��������ʵ���֮��Ϊ________��
(3)0.1mol lL-1A��Һ��0.1mol ?L-1B��Һ�������ϣ���Һ��________��,ԭ����(�����ӷ���ʽ˵��) ________________��
(4)�����ʵ�����D�����е�������(����ʵ�����������ͽ��ۣ���________________��
(5)pH��ͬ��A��D��H������Һ����ˮ�������c(OH��)�Ĵ�С��ϵ��(��A��D��H��ʾ) : ____________��
(6)��һ������Fe��FeO��Fe3O4�Ļ�����У�����1mol? L��1 A����Һ100 mL��ǡ��ʹ�����ȫ���ܽ⣬�ҷų�336mL(��״����)�����壬��������Һ�м���KSCN��Һ����Һ��ɫ���֣���ȡͬ������Fe��FeO��Fe3O4��������1 mol ? L��1 H����Һ,Ҳǡ��ʹ�����ȫ���ܽ⣬����������Һ�м���KSCN��Һ,��ҺҲ��ɫ����,���������H��Һ�������________��
��ÿ��2�֣����һ��ÿ��3�֣�����15�֣�
��1��2NH4Cl��Ca(OH)2 CaCl2��2H2O��2NH3�� ��2��2:3
CaCl2��2H2O��2NH3�� ��2��2:3
��3�����ԣ�NH4����H2O NH3��H2O��H��
NH3��H2O��H��
��4��ȡһ֧�Թܼ���������D���壬Ȼ�����������NaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤��D�����к���NH4��
��5��D��A��H ��6��110ml
��1��2NH4Cl��Ca(OH)2
 CaCl2��2H2O��2NH3�� ��2��2:3
CaCl2��2H2O��2NH3�� ��2��2:3��3�����ԣ�NH4����H2O
 NH3��H2O��H��
NH3��H2O��H����4��ȡһ֧�Թܼ���������D���壬Ȼ�����������NaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤��D�����к���NH4��
��5��D��A��H ��6��110ml
���������CΪ����Һ�壬��C������ˮ��ͨ��״���¼ס��ҡ���������Ϊ���嵥�ʣ�����Ͷ�Ӧ����������������A��B���ǻ�����Ҷ������塣����ΪA��B���Է�Ӧ����D��D��F��Ӧ����B��C��I���Ҹ÷�Ӧ����Ҫ��ʵ������ȡ����ķ�Ӧ���ɴ˿����Ʋ⣬�÷�Ӧ��ʵ������ȡ�����ķ�Ӧ�����Զ�������������������B�ܺ�������Ӧ����ˮ��E������B�ǰ����������ǵ�����A���Ȼ��⣬����������D���Ȼ�泥�F���������ƣ�I���Ȼ��ơ�E��NO��G��NO2��NO2����ˮ���������NO����H�����ᡣ
��1����Ӧ�ܵĻ�ѧ����ʽΪ2NH4Cl��Ca(OH)2
 CaCl2��2H2O��2NH3����
CaCl2��2H2O��2NH3������2��������NO��Ӧ���ɰ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ4NH3��6NO��5N2��6H2O�����л�ԭ���ǰ�������������NO�����������������Ҳ�ǻ�ԭ������ڻ�ԭ���������������ʵ���֮����2:3�����Ը÷�Ӧ���������뻹ԭ��������ʵ���֮��ҲΪ2:3��
��3��0.1mol lL-1A��Һ��0.1mol ?L-1B��Һ��������ǡ�÷�Ӧ�����Ȼ�泥���Һ��NH4��ˮ�⣬������Һ�����ԣ���Ӧ�����ӷ���ʽΪNH4����H2O
 NH3��H2O��H����
NH3��H2O��H������4��D���Ȼ�泥���������NH4����NH4���ܺͼӦ���ɰ�������˿���ͨ�����鰱����֤�������к���NH4����������ȷ�IJ�����ȡһ֧�Թܼ���������D���壬Ȼ�����������NaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤��D�����к���NH4����
��5����������ᶼ��������ˮ�ĵ��룬�ڶ���Ũ����ͬ�������¶�ˮ�����Ƴ̶���ͬ���Ȼ����ǿ�������Σ�����ˮNH4��ˮ�⣬�ٽ�ˮ�ĵ��룬����pH��ͬ��A��D��H������Һ����ˮ�������c(OH��)�Ĵ�С��ϵ��D��A��H��
��6����Һ�о�û�г��ֺ�ɫ������Һ�е����ʷֱ����Ȼ������������������Ҷ��ߵ����ʵ�������
 ��0.05mol��336ml�����������������ʵ�����0.336L��22.4L/mol��0.015mol������ݵ���ת����ȿ�֪������ԭ����������ʵ�����
��0.05mol��336ml�����������������ʵ�����0.336L��22.4L/mol��0.015mol������ݵ���ת����ȿ�֪������ԭ����������ʵ����� ��0.01mol�����Ը��ݵ�ԭ���غ��֪����������ʵ�����0.05mol��2��0.01mol��0.11mol���������������0.11mol��1mol/L��0.11L��110ml��4�����顢����ˮ�⡢���������ˮ����ƽ���Ӱ���Լ�������ԭ��Ӧ���йؼ����
��0.01mol�����Ը��ݵ�ԭ���غ��֪����������ʵ�����0.05mol��2��0.01mol��0.11mol���������������0.11mol��1mol/L��0.11L��110ml��4�����顢����ˮ�⡢���������ˮ����ƽ���Ӱ���Լ�������ԭ��Ӧ���йؼ����
��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
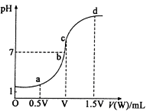
 ����3�֣�����Է�����������Ϊ158��160��162�������ַ��ӵ����ʵ���֮����7��10��7�����н�����ȷ���� ( )
����3�֣�����Է�����������Ϊ158��160��162�������ַ��ӵ����ʵ���֮����7��10��7�����н�����ȷ���� ( )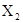 ��ƽ����Է�������Ϊ159
��ƽ����Է�������Ϊ159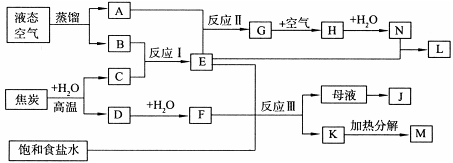
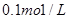 ��NaOH��Һ�ζ�
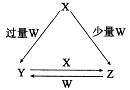
��NaOH��Һ�ζ�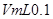 mo1/L HA��Һ���ζ�������ͼ��ʾ����a��b��c��d�ĵ���Һ��ˮ�ĵ���̶������� �㣻a����Һ������Ũ�ȵĴ�С˳��Ϊ ������c����Һ���Թ��У��ٵμ�0.1mo1/L���������ԣ���ʱ��Һ�г�H+��OH���� ������Ũ�ȵĴ�С˳��Ϊ ��
mo1/L HA��Һ���ζ�������ͼ��ʾ����a��b��c��d�ĵ���Һ��ˮ�ĵ���̶������� �㣻a����Һ������Ũ�ȵĴ�С˳��Ϊ ������c����Һ���Թ��У��ٵμ�0.1mo1/L���������ԣ���ʱ��Һ�г�H+��OH���� ������Ũ�ȵĴ�С˳��Ϊ ��