��Ŀ����
A��B��C��D�����ֳ������л�����У�A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L��������Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־֮һ��D�ķ���ʽΪC2H4O2��B��D��Ũ����ͼ��ȵ������·�����Ӧ�����ɵ��л������������ζ��A��B��C��D��һ�������µ�ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ���
�ش��������⣺
��1��ָ�����з�Ӧ�����ͣ�
������______��������______��
��2��A��B��D�У��Ȳ���ʹ���Ը��������Һ��ɫ���ֲ���ʹ��ˮ��ɫ����______������������ԭ�Ӿ���ͬһ��ƽ���ϵ���______��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
�ڣ�______
���𰸡�������A��B��C��D�����ֳ������л�����У�A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L��Ħ������=1.25g/L×22.4L/mol=28g/mol��������Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־֮һ����AΪCH2=CH2��A��ˮ�����ӳɷ�Ӧ����B����BΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��DΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��������������CH3COOCH2CH3�����ݴ˽��
����⣺A��B��C��D�����ֳ������л�����У�A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L��Ħ������=1.25g/L×22.4L/mol=28g/mol��������Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־֮һ����AΪCH2=CH2��A��ˮ�����ӳɷ�Ӧ����B����BΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��DΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��������������CH3COOCH2CH3����
��1����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ�����Ҵ�����������������ȩ��
�ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��2��A��B��D�У���ϩ����C=C˫�����ܱ����Ը�����������������巢���ӳɷ�Ӧ����ʹ���Ը��������Һ��ɫ������ʹ��ˮ��ɫ���Ҵ��ܱ��ܱ����Ը������������ʹ���Ը��������Һ��ɫ������Ȳ���ʹ���Ը��������Һ��ɫ���ֲ���ʹ��ˮ��ɫ��
�Ҵ��������к��м������м���������ṹ������ԭ�Ӳ����ܹ��棬��ϩΪƽ��ṹ������ԭ�Ӵ���ͬһƽ�棬
�ʴ�Ϊ��CH3COOH��CH2=CH2��
��3����Ӧ�����Ҵ���Cu��Ag�����������·���������ӦCH3CHO����Ӧ����ʽΪ��2CH3CH2OH+O2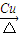 2CH3CHO+2H2O��
2CH3CHO+2H2O��
B+D��Ӧ���������Ҵ�����������Ӧ����������������Ӧ����ʽΪ��CH3CH2OH+CH3COOH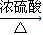 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��2CH3CH2OH+O2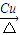 2CH3CHO+2H2O��CH3CH2OH+CH3COOH
2CH3CHO+2H2O��CH3CH2OH+CH3COOH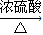 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
���������⿼���л����ƶϡ�ϩ�봼��ȩ������֮���ת����ϵ�ȣ��ѶȲ���ע�����֪ʶ���������գ�
����⣺A��B��C��D�����ֳ������л�����У�A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L��Ħ������=1.25g/L×22.4L/mol=28g/mol��������Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־֮һ����AΪCH2=CH2��A��ˮ�����ӳɷ�Ӧ����B����BΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��DΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��������������CH3COOCH2CH3����
��1����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ�����Ҵ�����������������ȩ��
�ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��2��A��B��D�У���ϩ����C=C˫�����ܱ����Ը�����������������巢���ӳɷ�Ӧ����ʹ���Ը��������Һ��ɫ������ʹ��ˮ��ɫ���Ҵ��ܱ��ܱ����Ը������������ʹ���Ը��������Һ��ɫ������Ȳ���ʹ���Ը��������Һ��ɫ���ֲ���ʹ��ˮ��ɫ��
�Ҵ��������к��м������м���������ṹ������ԭ�Ӳ����ܹ��棬��ϩΪƽ��ṹ������ԭ�Ӵ���ͬһƽ�棬
�ʴ�Ϊ��CH3COOH��CH2=CH2��
��3����Ӧ�����Ҵ���Cu��Ag�����������·���������ӦCH3CHO����Ӧ����ʽΪ��2CH3CH2OH+O2
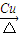 2CH3CHO+2H2O��
2CH3CHO+2H2O��B+D��Ӧ���������Ҵ�����������Ӧ����������������Ӧ����ʽΪ��CH3CH2OH+CH3COOH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O���ʴ�Ϊ��2CH3CH2OH+O2
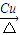 2CH3CHO+2H2O��CH3CH2OH+CH3COOH
2CH3CHO+2H2O��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O�����������⿼���л����ƶϡ�ϩ�봼��ȩ������֮���ת����ϵ�ȣ��ѶȲ���ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
a��b��c��d�����ֶ�����Ԫ�أ�a��b��dͬ���ڣ�c��dͬ���壮a��ԭ�ӽṹʾ��ͼΪ ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ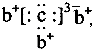 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������
 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ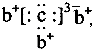 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
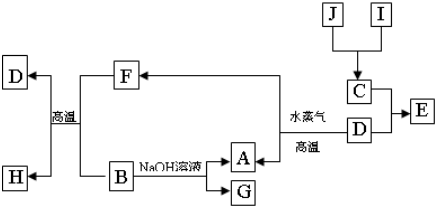
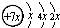 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
 Al��OH��3+OH-
Al��OH��3+OH-