��Ŀ����
����Ŀ��ij����I��һ�ֺϳ�·�����£�

�ش��������⣺
(1)C������Ϊ______________;H����������������______________��
(2)B��C�ķ�Ӧ������______________;д��I�Ľṹ��ʽ��______________��
(3)G���������______________��ԭ�ӹ�ƽ�档
(4)д��A��B�Ļ�ѧ����ʽ��____________________________��
(5)J��I��ͬ���칹��,ͬʱ��������������J�Ľṹ��_______�֡����У�һ�ֺ˴Ź���������6�����ҷ�����֮��Ϊ1��1��1��2��2��3�Ľṹ��ʽΪ______________��
����ʹ������Ȼ�̼��Һ��ɫ
������̼��������Һ��Ӧ����CO2
�����ڷ����廯����ұ����ϵ�һ�ȴ���ֻ��2��
(6)������������,����ϩ��OHC-CHOΪԭ�Ϻϳ�HOOCCH=CHCOOH,��ƺϳ�·�ߣ�___________��
���𰸡����״�̼̼˫�����Ȼ�ȡ����Ӧ(��ˮ�ⷴӦ)![]() 18
18![]() +Cl2
+Cl2![]()
![]() +HCl6
+HCl6![]() ��
��![]() CH2=CH2
CH2=CH2![]() CH3CHO
CH3CHO![]() OHCCH=CHCHO
OHCCH=CHCHO![]() HOOCCH=CHCOOH
HOOCCH=CHCOOH
��������
�ɺϳ�·���Լ��л���Ӧ�����ɵ�AΪ![]() ��BΪ
��BΪ![]() ��CΪ
��CΪ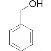 ��HΪ
��HΪ![]() ��IΪ
��IΪ![]() ���ݴ˷����ɵý��ۡ�
���ݴ˷����ɵý��ۡ�
(1)CΪ ������Ϊ���״���HΪ
������Ϊ���״���HΪ![]() �������к���̼̼˫�����Ȼ����ֹ����ţ��ʴ�Ϊ�����״���̼̼˫�����Ȼ�(2) B��C�ķ�ӦΪ±������ˮ�ⷴӦ��Ҳ��ȡ����Ӧ��I�Ľṹ��ʽ��
�������к���̼̼˫�����Ȼ����ֹ����ţ��ʴ�Ϊ�����״���̼̼˫�����Ȼ�(2) B��C�ķ�ӦΪ±������ˮ�ⷴӦ��Ҳ��ȡ����Ӧ��I�Ľṹ��ʽ��![]() ���ʴ�Ϊ��ȡ����Ӧ(��ˮ�ⷴӦ)��
���ʴ�Ϊ��ȡ����Ӧ(��ˮ�ⷴӦ)��![]() (3) G�����еı�����̼̼˫����ȩ������ƽ��ṹ����G���������е�ԭ�Ӷ��п��ܹ��棬�ʴ�Ϊ18��(4) д��A��B�ķ�ӦΪ�ױ������ϵ�һ��ȡ�����ʴ�Ϊ��
(3) G�����еı�����̼̼˫����ȩ������ƽ��ṹ����G���������е�ԭ�Ӷ��п��ܹ��棬�ʴ�Ϊ18��(4) д��A��B�ķ�ӦΪ�ױ������ϵ�һ��ȡ�����ʴ�Ϊ��![]() +Cl2
+Cl2![]()
![]() +HCl ��(5)����ʹ������Ȼ�̼��Һ��ɫ˵�������к���̼̼˫����������̼��������Һ��Ӧ����CO2˵�����ӽṹ�к����Ȼ��������ڷ����廯����ұ����ϵ�һ�ȴ���ֻ��2��˵�����ӽṹ�к��д��ڶ�λ������ȡ������������Ϸ����ɵ÷��Ͻṹ�ķ�����6�ֽṹ���ʴ�Ϊ��6��(6) �����������̿ɵ���ϩ��OHC-CHOΪԭ�Ϻϳ�HOOCCH=CHCOOH��·��Ϊ��CH2=CH2
+HCl ��(5)����ʹ������Ȼ�̼��Һ��ɫ˵�������к���̼̼˫����������̼��������Һ��Ӧ����CO2˵�����ӽṹ�к����Ȼ��������ڷ����廯����ұ����ϵ�һ�ȴ���ֻ��2��˵�����ӽṹ�к��д��ڶ�λ������ȡ������������Ϸ����ɵ÷��Ͻṹ�ķ�����6�ֽṹ���ʴ�Ϊ��6��(6) �����������̿ɵ���ϩ��OHC-CHOΪԭ�Ϻϳ�HOOCCH=CHCOOH��·��Ϊ��CH2=CH2![]() CH3CHO
CH3CHO![]() OHCCH=CHCHO
OHCCH=CHCHO![]() HOOCCH=CHCOOH��
HOOCCH=CHCOOH��

 �߽�������ϵ�д�
�߽�������ϵ�д�����Ŀ��Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3������������MgO��SiO2�����ʣ����Ʊ��������������£�

�ش��������⣺
��1������1Ϊ_______________������AΪ_________________������Һ���м���˫��ˮ��������___________________________________��
��2���������������Ҫ��TiOCl42����ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ__________��
��3��TiO2��xH2O������˫��ˮ����ˮ��Ӧ40 min����ʵ�������±���ʾ��
�¶�/�� | 30 | 35 | 40 | 45 | 50 |
TiO2��xH2Oת����% | 92 | 95 | 97 | 93 | 88 |
����40��ʱTiO2��xH2Oת������ߵ�ԭ��_______________________________��
��4��������Һ�ڡ���c(Mg2+)=0.02 mol/L������˫��ˮ�����ᣨ����Һ�������1������ʹFe3+ǡ�ó�����ȫ����Һ��c(Fe3+)=1��10-5 mol/L����ʱ�Ƿ���Mg3(PO4)2�������ɣ�_____________________________________________����ʽ���㣩��FePO4��Mg3(PO4)2��Ksp�ֱ�Ϊ1.3��10-22��1.0��10-24��