��Ŀ����
6������ʵ��IJ��������õ��Լ��д������DEFA��ʵ������ȡ��ϩ����ʱ�����¶ȼƵ�ˮ�������Һ�����£�
B�����þƾ�ϴ��մ�б��ӵ��Թܣ�
C��֤������CH2=CH-CHO�Ⱥ���ȩ���ֺ���
 ����������Һ�м���������������Һ���ȣ���ַ�Ӧ���ټ�����������ˮ��
����������Һ�м���������������Һ���ȣ���ַ�Ӧ���ټ�����������ˮ��D������������Һʱ������������Һ��ε��백ˮ�У�
E�����л��б��ӣ���Ũ��ˮ��Ȼ���Һ��
F����Ũ��ˮϴ������������Ӧ���Թܣ�
���� A��ʵ������ȡ��ϩ����ʱ���ⶨ��ӦҺ���¶ȣ�
B�����������ھƾ���
C��������Һ�м���������������Һ���ȣ�������-CHO��
D������������Һʱ�������ɵij���ǡ���ܽ⼴�ɣ�
E���塢���屽�Ӿ������ڱ���
F��Ag�백ˮ����Ӧ��
��� �⣺A��ʵ������ȡ��ϩ����ʱ���ⶨ��ӦҺ���¶ȣ����¶ȼƵ�ˮ�������Һ�����£���A��ȷ��
B�����������ھƾ�������þƾ�ϴ��մ�б��ӵ��Թܣ���B��ȷ��
C��������Һ�м���������������Һ���ȣ�������-CHO���ټ�����������ˮ����ˮ��ɫ����̼̼˫������C��ȷ��
D������������Һʱ�������ɵij���ǡ���ܽ⼴�ɣ�������������Һ����ε��백ˮ����D����
E���塢���屽�Ӿ������ڱ������ܳ��ӣ�Ӧ��NaOH��Һ����Һ���ӣ���E����
F��Ag�백ˮ����Ӧ��Ӧ��������ϴ������������Ӧ���Թܣ���F����
�ʴ�Ϊ��DEF��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰�л�������ʡ��Ʊ������������ᴿ�������ż���ȣ��������ʵ����ʡ���Ӧԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14�����и������ʣ�һ����Ϊͬϵ����ǣ�������
| A�� | ����ͬһͨʽ������ | |
| B�� | ������ͬ�����ŵ����� | |
| C�� | ��Է����������14����14�ı��������� | |
| D�� | ͨʽΪCnH2n+2����Cԭ��������ȵ����� |
1�����ֶ�����Ԫ�ص�����aXm+��bYn+��cZn-��dRm-�����Ǿ�����ͬ�ĵ��Ӳ�ṹ����m��n��������������ȷ���ǣ�������
��a-b=n-m
��Ԫ�ص�ԭ������a��b��c��d
��Ԫ�طǽ�����Z��R
������������Ӧˮ����ļ���X��Y
�ݻ�ԭ��Rm-��Zn-
��Xһ��������ˮ��Ӧ��
��a-b=n-m
��Ԫ�ص�ԭ������a��b��c��d
��Ԫ�طǽ�����Z��R
������������Ӧˮ����ļ���X��Y
�ݻ�ԭ��Rm-��Zn-
��Xһ��������ˮ��Ӧ��
| A�� | ֻ�Т���ȷ | B�� | �٢ڢۢ���ȷ | C�� | �٢ڢۢ���ȷ | D�� | �ڢۢ���ȷ |
11���ܣ�Cu�����仯�����ڹ�ҵ���й㷺Ӧ�ã�Ϊ��ij��ҵ�����л����ܣ�ijѧ������������£������к���Al��Li��Co2O3��Fe2O3�����ʣ���

��֪�����ֽ��������γ��������������pH���±���
��ش�
��1��������еõ�������Һ�ķ�Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��2��д���������Co2O3��Na2CO3��Ӧ�����ӷ���ʽ��Co2O3+SO32-+4H+=Co2++SO42-+2H2O��
��3�������������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽClO3-+6H++5Cl-=3Cl2��+3H2O�����У�Na2CO3��Һ�������ǵ�����ҺPHʹ�����ӳ�����ȫת��ΪFe��OH��3��
��4���ڿ����м��Ȳ����ܾ��壨CoC2O4•2H2O����Ʒ��Ҫ�õ�����Ҫ������������5.49g�þ������ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ����������±���
���ⶨ��210��290������в���������ֻ��CO2���˹��̷�����Ӧ�Ļ�ѧ����ʽ��3CoC2O4+2O2$\frac{\underline{\;210��-290��\;}}{\;}$Co3O4+6CO2��[M ��CoC2O4•2H2O��=183 g/mol]
��5���ӷ�Ӧ��Ļ�����еõ������ܾ��壬��Ծ������ϴ�ӣ�֤�������Ѿ�ϴ�Ӹɾ��IJ�����������ȡ���һ��ϴ��Һ���Թ��У����뼸��ϡ�����ữ����������Һ�����ް�ɫ�������ɣ�˵���Ѿ�ϴ����

��֪�����ֽ��������γ��������������pH���±���
| Fe3+ | Co2+ | Co3+ | Al2+ | |
| pH����ʼ������ | 1.9 | 7.15 | -0.23 | 3.4 |
| pH����ȫ������ | 3.2 | 9.15 | 1.09 | 4.7 |
��1��������еõ�������Һ�ķ�Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��2��д���������Co2O3��Na2CO3��Ӧ�����ӷ���ʽ��Co2O3+SO32-+4H+=Co2++SO42-+2H2O��
��3�������������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽClO3-+6H++5Cl-=3Cl2��+3H2O�����У�Na2CO3��Һ�������ǵ�����ҺPHʹ�����ӳ�����ȫת��ΪFe��OH��3��
��4���ڿ����м��Ȳ����ܾ��壨CoC2O4•2H2O����Ʒ��Ҫ�õ�����Ҫ������������5.49g�þ������ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ����������±���
| �¶ȷ�Χ/�� | ��������/g |
| 150��210 | 4.41 |
| 290��320 | 2.41 |
| 890��920 | 2.25 |
��5���ӷ�Ӧ��Ļ�����еõ������ܾ��壬��Ծ������ϴ�ӣ�֤�������Ѿ�ϴ�Ӹɾ��IJ�����������ȡ���һ��ϴ��Һ���Թ��У����뼸��ϡ�����ữ����������Һ�����ް�ɫ�������ɣ�˵���Ѿ�ϴ����
15����һ�ܱ������У��������۲�����һ������CO���壬һ�������·�����Ӧ��Ni��s��+4CO��g��?���ɣ�������4��g������֪�÷�Ӧ��25���80��ʱ��ƽ�ⳣ���ֱ�Ϊ5��104��2������˵����ȷ���ǣ�������
| A�� | ���º����£����������ٳ�������Ni��CO��4��g��������ƽ��ʱ��Ni��CO��4�ٷֺ��������� | |
| B�� | ��80��ʱ�����ijʱ��Ni��CO��4��COŨ�Ⱦ�Ϊ0.5 mol•L-1�����ʱv��������v���棩 | |
| C�� | ���º�ѹ�£����������ٳ���������Ar������ƽ�⽫�����ƶ� | |
| D�� | ��������Ni��CO��4��g���ķ�ӦΪ���ȷ�Ӧ |

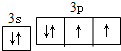
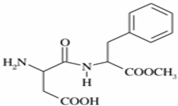 ��˹����APM����һ����ȸߡ�ζ���������͵���ζ������ṹ��ʽ��ͼ��ʾ��
��˹����APM����һ����ȸߡ�ζ���������͵���ζ������ṹ��ʽ��ͼ��ʾ��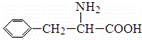 �Ǻϳ�APM��ԭ��֮һ�����������һ�ֺϳ�;����ͼ��ʾ��
�Ǻϳ�APM��ԭ��֮һ�����������һ�ֺϳ�;����ͼ��ʾ��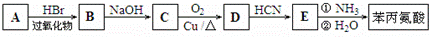
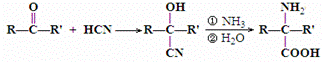
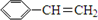

 ��
��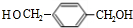 ��
��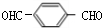 +2H2O��
+2H2O��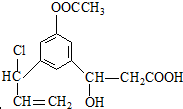 ������ӦΪ�٢ڢۢܢݢ�
������ӦΪ�٢ڢۢܢݢ�