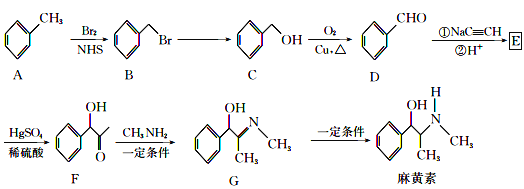
��Ŀ����
����Ŀ��NH4NO3��Һ���ȿɷ����ֽⷴӦ��NH4NO3![]() N2����HNO3��H2O(δ��ƽ)����NA��ʾ�����ӵ�������ֵ������˵����ȷ����(����)
N2����HNO3��H2O(δ��ƽ)����NA��ʾ�����ӵ�������ֵ������˵����ȷ����(����)
A.�ֽ�ʱÿ����2.24 L(��״��)N2��ת�Ƶ��ӵ���ĿΪ0.6NA
B.2.8 g N2�к��й��õ��ӶԵ���ĿΪ0.3NA
C.56 g Fe��������Ũ���ᷴӦ����NO2���ӵ���ĿΪ3NA
D.0.1 mol��L��1 NH4NO3��Һ�У�NH4+����ĿС��0.1NA
���𰸡�B
��������
NH4NO3��Һ���ȿɷ����ֽⷴӦ��5NH4NO3![]() 4N2����2HNO3��9H2O��
4N2����2HNO3��9H2O��
A. �÷�Ӧ���ڹ��з�Ӧ��NH4+�У�3�۵ĵ�Ԫ������Ϊ0�ۣ���������NO3���У�5�۵ĵ�Ԫ�ؽ���Ϊ0�ۣ�����ԭ���������ĵ�ԭ���뱻��ԭ�ĵ�ԭ�ӵ����ʵ���֮��Ϊ5��3��ÿ����4mol N2��ת��15mol���ӣ����ԣ�������0.1 mol N2ʱ��ת�Ƶ��ӵ����ʵ���Ϊ![]() ��0.1mol��0.375 mol��ת�Ƶĵ�����Ϊ0.375NA��A����
��0.1mol��0.375 mol��ת�Ƶĵ�����Ϊ0.375NA��A����
B. N2�ĽṹʽΪN��N��1�� N2���Ӻ���3�Թ��õ��ӣ�2.8 g N2�����ʵ���Ϊ0.1mol�����еĹ��õ��Ӷ���Ϊ0.3NA��B��ȷ��
C. Ũ�������ǿ�����ԣ��ɽ�������Ϊ��3�ۣ���������ԭΪNO2��56g Fe�����ʵ���Ϊ1mol����ʧȥ3mol���ӣ����ԣ���Ӧ����3mol NO2������һ����NO2ת��ΪN2O4���������ɵ�NO2����������3NA��C����
D. ��֪�������Һ�����ʵ���Ũ�ȣ���δ��֪��Һ�����������ȷ����������淋����ʵ����������㺬��NH4+����Ŀ��D����
�ʴ�Ϊ��B��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д� �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�����Ŀ����.�����£�Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��pH�����ʾ��
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | Na2SO4 |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 7.0 |
��ش��������⣺
(1)����������Һ�У�ˮ�ĵ���̶���С����________(�ѧʽ)��
(2)����������ˮ�д������Ũ�ȣ�������ˮ�м����ϱ��е�������_____(��дһ�����ʼ���)��
��.�����£���100 mL 0.2 mol��L��1�İ�ˮ����μ���0.2 mol��L��1�����ᣬ������Һ��pH����Һ��NH4+��NH3��H2O�����ʵ���������������������Ĺ�ϵ��ͼ��ʾ��
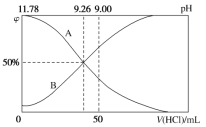
(3)����ͼ��ش��������⡣
�ٱ�ʾNH3��H2OŨ�ȱ仯��������__________(����A������B��)��
��NH3��H2O�ĵ��볣��Ϊ______________��
�۵������������Ϊ50 mLʱ����Һ��c(NH4+)��c(NH3��H2O)=______ mol��L��1(�����ּ���ʽ��ʾ)��
��.��֪��Ag����SCN��=AgSCN��(��ɫ)��ʵ���ҿ�ͨ�����¹��̲ⶨ������������Ʒ�Ĵ���(���ʲ����뷴Ӧ)��
(4)��ȡ2.000 g�Ʊ�����������Ʒ����ˮ�ܽ⣬���ݵ�100 mL����Һ���ƹ��������õIJ����������ձ������������________��
(5)ȷ��ȡ25.00 mL��Һ���ữ����뼸����立�[NH4Fe(SO4)2]��Һ��ָʾ��������0.100 mol��L��1 NH4SCN����Һ�ζ����ζ��յ��ʵ������Ϊ____________���յ�ʱ���ı���Һ25mL����������Ʒ����Ϊ_______��
����Ŀ��PCl3���ij����Ȼ�������ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
�۵�/�� | �е�/�� | �ܶ�/ g��mL��1 | ���� | |
���� | 44.1 | 280.5 | 1.82 | 2P��3Cl2(����) |
PCl3 | ��112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 |
(һ)�Ʊ�
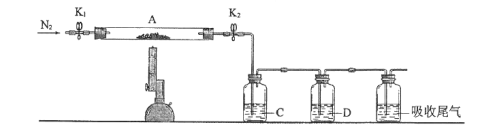
��ͼ��ʵ�����Ʊ�PCl3��װ��(����������ʡ��)��

(1)�����ҵ�������________�����У�������ˮ��ˮ�����ӵĽӿڱ����________��(����a����b��)
(2)ʵ�����Ʊ�Cl2�����ӷ���ʽ___________________________��ʵ������У�Ϊ����PCl5�����ɣ�Ӧ����____________��
(3)��ʯ�ҵ����ã�һ�Ƿ�ֹ�����е�ˮ���������ʹPCl3ˮ�⣬Ӱ���Ʒ�Ĵ��ȣ�����_________��
(4)����������ͨ�����Cl2֮ǰ��Ӧ��ͨ��һ��ʱ��CO2�ž�װ���еĿ�������Ŀ����________��
(��)����
�ⶨ��Ʒ��PCl3���ȵķ������£�Ѹ�ٳ�ȡ4.100 g��Ʒ��ˮ����ȫ�����500 mL��Һ��ȡ��25.00 mL���������0.100 0 mol��L��1 20.00 mL����Һ����ַ�Ӧ������0.100 0 mol��L��1 Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����12.00 mL Na2S2O3��Һ��
��֪��H3PO3��H2O��I2===H3PO4��2HI��I2��2Na2S2O3===2NaI��Na2S4O6������ⶨ������û��������Ӧ��
(5)�����������ݣ��ò�Ʒ��PCl3(��Է�������Ϊ137.5)����������Ϊ________�����ζ��յ�ʱ���Ӷ�������PCl3����������________(����ƫ������ƫС��������Ӱ����)��
(��)̽��
(6)���ʵ��֤��PCl3���л�ԭ�ԣ�_____________________________________��(��ѡ�Լ��У�����ˮ��ϡ���ᡢ��ˮ������)
����Ŀ���й���ͳ�Ļ��������������Ŵ������м����˹Ŵ���ѧ�о��ɹ������г�����ʫ�Ķ�Ӧ�Ļ�ѧ֪ʶ��ȷ����
������ʫ�ļ��� | ��ѧ֪ʶ | |
A | ����Ϫ��̸���жԱ����ļ��أ��������Լ���Ϊ�У�����Ϊ���ɣ����������ۡ� | ���ĺϽ�Ӳ�ȱȴ����Ĵ��۵�ȴ����ĸ� |
B | �����ݸ�Ŀʰ�š��ж�ǿˮ�ļ��أ��������ң���ʴ�����ˮ��ǿ��Ω������ʢ�� | ǿˮΪ����� |
C | ���칤����м��أ�����ҩ����Ϊ��������Ϊ���� | ��ָ������ƣ���ָ��������� |
D | ��Ȫ�ݸ�־���м��أ������˻�����Ϊլ���ǣ�ǽ��ѹ�ǣ�ȥ�����ǰף�������Ч֮ | �ǰĹ��̷����˻�ѧ�仯 |
A. AB. BC. CD. D
����Ŀ���Ȼ���ͭ��CuCl���������л��ϳɹ�ҵ�еĴ������ڿ�����Ѹ�ٱ����������ɫ������ֽ��ɺ�ɫ����ͼ�ǹ�ҵ��������ӡˢ��·�ķ�Һ����Fe3+��Cu2+��Fe2+��Cl������CuCl�����̣�
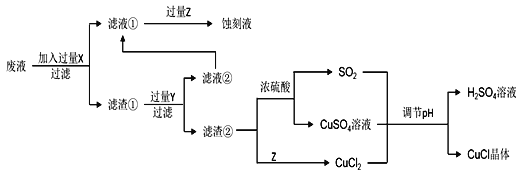
����������Ϣ�ش��������⣺
��1������������X�Ļ�ѧʽΪ____��
��2��д������CuCl�����ӷ���ʽ��____��
��3��ʵ��̽��pH��CuCl���ʵ�Ӱ�������ʾ��
pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
CuCl����/% | 70 | 90 | 82 | 78 | 75 | 72 | 70 |
����CuCl�������pHΪ____����pH�ϴ�ʱCuCl���ʱ��ԭ����____������pHʱ��___��������������������������ͬpH������������ᣬ������____��
��4���Ȼ���ͭ�Ķ���
�ٳ�ȡ��Ʒ0.25g������FeCl3��Һ����ƿ�У�����ܽ⡣
����0.10mol��L1���������Һ�ζ�����֪��CuCl+FeCl3=CuCl2+FeCl2��Fe2++Ce4+=Fe3++Ce3+������ƽ��ʵ���������ƽ��ʵ�������ܳ���1%����
ƽ��ʵ����� | 1 | 2 | 3 |
0.25g��Ʒ�������������Һ�������mL�� | 24.35 | 24.05 | 23.95 |
����Ʒ��CuCl�Ĵ���Ϊ_____�������������λ��Ч���֣���
��5����CuClˮ�����ȷֽ�ɵõ�����Cu2O����һ��CuClˮ������ӷ���ʽΪ��CuCl(s)��H2O(l)![]() CuOH(s)��Cl (aq)��H+(aq)���ڶ���CuOH�ȷֽ�Ļ�ѧ����ʽΪ____����һ��CuClˮ�ⷴӦ��ƽ�ⳣ��K����¶���KW��Ksp(CuOH)��Ksp(CuCl)�Ĺ�ϵΪK��____��
CuOH(s)��Cl (aq)��H+(aq)���ڶ���CuOH�ȷֽ�Ļ�ѧ����ʽΪ____����һ��CuClˮ�ⷴӦ��ƽ�ⳣ��K����¶���KW��Ksp(CuOH)��Ksp(CuCl)�Ĺ�ϵΪK��____��