��Ŀ����
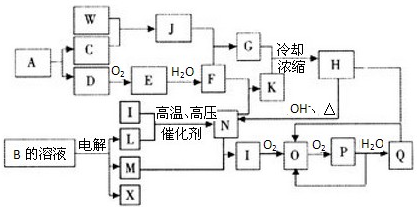
��ͼ��һЩ���ʼ��ת����ϵͼ��ͼ���������ʺ��е�Ԫ����ֻ��һ�ֲ��Ƕ�����Ԫ�أ����з�Ӧ������P��Һ�е�ˮ�Լ����ַ�Ӧ��һЩ������δ�����C��D��E��KΪ���ʣ�NΪ���Ի����J��������Ԫ����ɵĺ�SiC��ͬ�������͵����Ͳ��ϣ���J��SiC������ͬ�ļ۵�������ԭ������I��������Ԫ����ɵ�ǿ���Ӧ�����ڼ���һ�ֳ������л������ţ���Ӧ�ݡ������ڹ�ҵ������B��

�ش��������⣺
��1��д���������ʵĻ�ѧʽ��G ��I ��
��2��д����Ӧ�١��ߵ����ӷ���ʽ��
�� ��
�� ��
��3��J��SiC���������Ϸ�ĩ����һ�����ģ���ɵ�·�����ɢ�Ȳ��ϡ���Ӧ�����ձ���ѧ���о������Ʊ����������Ϸ�ĩ������;������֪��Ӧ����n(F)��n(E)��n(J)��n(SiC)��n(K)��1��2��4��1��3��д����Ӧ�ڵĻ�ѧ����ʽ ��
��1�� NO��Ag(NH3)2OH
��2��4Ag����2H2O![]() 4Ag��4H����O2����CO2��2AlO2����3H2O��2Al(OH)3����CO32��
4Ag��4H����O2����CO2��2AlO2����3H2O��2Al(OH)3����CO32��
��3�� Al4SiC4��2N2![]() 4AlN��SiC��3C
4AlN��SiC��3C

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�

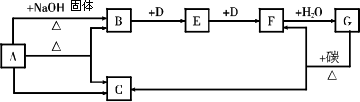
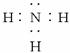
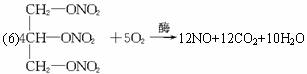 ����������Һ��ø�������£����������ͷų�D���ӣ�ͬʱ���ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________��
����������Һ��ø�������£����������ͷų�D���ӣ�ͬʱ���ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________��