��Ŀ����
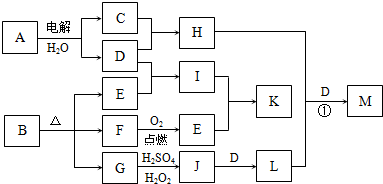
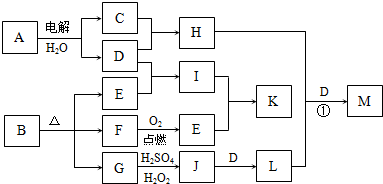
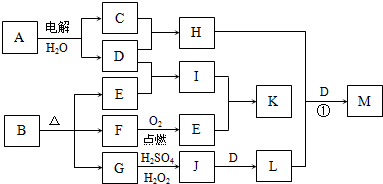
��ͼΪһЩ����֮���ת����ϵ�����в��ַ�Ӧ�з�Ӧ���������δ��ȫ����֪A��H��I��K��Ϊ��ͥ�����еij������ʣ�����A��ʳƷ��ζ����H������������Ч�ɷ֣�I��K������ʳƷ���ݼ���B��һ���л����Σ�E��F�����������ʵ���֮��Ϊ1��1����G��Ϊ�����L�Ǻ��ɫ������
����������Ϣ���ش��������⣺
��1��E�ĵ���ʽ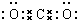
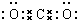 ��
��
��2����̼�������������������������з�̪��A�ı�����Һ����ͨ��Դһ��ʱ�������������Һ��ɫ�������ԭ����
��3��G��J�����ӷ���ʽΪ
��4����Ӧ����H�ܽ�L����������һ�ֺ�������M������ijԪ�صĻ��ϼ�Ϊ+6���������������ʣ���H��L��D�����ʵ���֮��Ϊ3��2��4����H+L+D��M�Ļ�ѧ����ʽΪ
��5����������ʵ���Ũ�ȵ�I��Kϡ��Һ��ʵ�鷽��Ϊ

����������Ϣ���ش��������⣺
��1��E�ĵ���ʽ
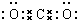
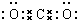
��2����̼�������������������������з�̪��A�ı�����Һ����ͨ��Դһ��ʱ�������������Һ��ɫ�������ԭ����
H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+��
H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+��
����3��G��J�����ӷ���ʽΪ
2FeO+H2O2+6H+=2Fe3++4H2O
2FeO+H2O2+6H+=2Fe3++4H2O
����4����Ӧ����H�ܽ�L����������һ�ֺ�������M������ijԪ�صĻ��ϼ�Ϊ+6���������������ʣ���H��L��D�����ʵ���֮��Ϊ3��2��4����H+L+D��M�Ļ�ѧ����ʽΪ
3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O
3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O
����5����������ʵ���Ũ�ȵ�I��Kϡ��Һ��ʵ�鷽��Ϊ
ȡI��K��ϡ��Һ���ֱ�μ�CaCl2��Һ�����ְ�ɫ������ΪI
ȡI��K��ϡ��Һ���ֱ�μ�CaCl2��Һ�����ְ�ɫ������ΪI
��������A��ʳƷ��ζ������AΪNaCl��H������������Ч�ɷ֣���HΪNaClO��GΪ�������H2SO4��H2O2����J��J��D��L��LΪ���ɫ��������LΪFe��OH��3��DΪNaOH��JΪFe2��SO4��3��GΪFeO����B�к���FeԪ�أ�CΪCl2��BΪ�л����Σ���һ������C��O��Ԫ�أ�E��FΪ�������Ͽ�ͼ����FΪCO��EΪCO2��IΪNa2CO3�������KΪNaHCO3��С�մ�������ʳƷ�ķ��ݼ���
��1��EΪCO2��Ϊ���ۻ����������C��Oԭ��֮����2�Թ��õ��Ӷԣ�
��2�����NaCl��Һ������������ԭ��Ӧ����ӦʽΪ��2H+-2e-�TH2����H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣻
��3��G��J�ķ�ӦΪFeO����Fe2��SO4��3�Ĺ��̣�H2O2���������ԣ���Ӧ�����ӷ���ʽΪ2FeO+H2O2+6H+=2Fe3++4H2O��
��4��H��L��D�ֱ�Ϊ��NaClO��Fe��OH��3��NaOH���������ʵ���֮��Ϊ3��2��4���������һ�ֺ�������M����ȷ��M�Ļ�ѧʽΪNa2FeO4���Դ���д��ѧ����ʽ��
��5��IΪNa2CO3�������KΪNaHCO3��С�մ�Na2CO3������CaCO3��Ϊ��ɫ�������ɼ���CaCl2��Һ����
��1��EΪCO2��Ϊ���ۻ����������C��Oԭ��֮����2�Թ��õ��Ӷԣ�
��2�����NaCl��Һ������������ԭ��Ӧ����ӦʽΪ��2H+-2e-�TH2����H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣻
��3��G��J�ķ�ӦΪFeO����Fe2��SO4��3�Ĺ��̣�H2O2���������ԣ���Ӧ�����ӷ���ʽΪ2FeO+H2O2+6H+=2Fe3++4H2O��
��4��H��L��D�ֱ�Ϊ��NaClO��Fe��OH��3��NaOH���������ʵ���֮��Ϊ3��2��4���������һ�ֺ�������M����ȷ��M�Ļ�ѧʽΪNa2FeO4���Դ���д��ѧ����ʽ��
��5��IΪNa2CO3�������KΪNaHCO3��С�մ�Na2CO3������CaCO3��Ϊ��ɫ�������ɼ���CaCl2��Һ����
����⣺A��ʳƷ��ζ������AΪNaCl��H������������Ч�ɷ֣���HΪNaClO��GΪ�������H2SO4��H2O2����J��J��D��L��LΪ���ɫ��������LΪFe��OH��3��DΪNaOH��JΪFe2��SO4��3��GΪFeO����B�к���FeԪ�أ�CΪCl2��BΪ�л����Σ���һ������C��O��Ԫ�أ�E��FΪ�������Ͽ�ͼ����FΪCO��EΪCO2��IΪNa2CO3�������KΪNaHCO3��С�մ�������ʳƷ�ķ��ݼ���
��1��EΪCO2��Ϊ���ۻ����������C��Oԭ��֮����2�Թ��õ��Ӷԣ�����ʽΪ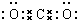 ���ʴ�Ϊ��
���ʴ�Ϊ��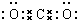 ��
��
��2�����NaCl��Һ������������ԭ��Ӧ����ӦʽΪ��2H+-2e-�TH2����H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+������Һ�ʼ��ԣ�
�ʴ�Ϊ��H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+����
��3��G��J�ķ�ӦΪFeO����Fe2��SO4��3�Ĺ��̣�H2O2���������ԣ���Ӧ�����ӷ���ʽΪ2FeO+H2O2+6H+=2Fe3++4H2O���ʴ�Ϊ��2FeO+H2O2+6H+=2Fe3++4H2O��
��4��H��L��D�ֱ�Ϊ��NaClO��Fe��OH��3��NaOH���������ʵ���֮��Ϊ3��2��4���������һ�ֺ�������M����ȷ��M�Ļ�ѧʽΪNa2FeO4������ʽΪ
3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O���ʴ�Ϊ��3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O��
��5��IΪNa2CO3�������KΪNaHCO3��С�մ�Na2CO3������CaCO3��Ϊ��ɫ�������ɼ���CaCl2��Һ���𣬳��ְ�ɫ������ΪNa2CO3��
�ʴ�Ϊ��ȡI��K��ϡ��Һ���ֱ�μ�CaCl2��Һ�����ְ�ɫ������ΪI��
��1��EΪCO2��Ϊ���ۻ����������C��Oԭ��֮����2�Թ��õ��Ӷԣ�����ʽΪ
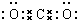 ���ʴ�Ϊ��
���ʴ�Ϊ��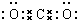 ��
����2�����NaCl��Һ������������ԭ��Ӧ����ӦʽΪ��2H+-2e-�TH2����H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+������Һ�ʼ��ԣ�
�ʴ�Ϊ��H+���������ϵõ����ӳ�ΪH2���Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹc��OH-����c��H+����
��3��G��J�ķ�ӦΪFeO����Fe2��SO4��3�Ĺ��̣�H2O2���������ԣ���Ӧ�����ӷ���ʽΪ2FeO+H2O2+6H+=2Fe3++4H2O���ʴ�Ϊ��2FeO+H2O2+6H+=2Fe3++4H2O��
��4��H��L��D�ֱ�Ϊ��NaClO��Fe��OH��3��NaOH���������ʵ���֮��Ϊ3��2��4���������һ�ֺ�������M����ȷ��M�Ļ�ѧʽΪNa2FeO4������ʽΪ
3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O���ʴ�Ϊ��3NaClO+2Fe��OH��3+4NaOH=2Na2FeO4+3NaCl+5H2O��
��5��IΪNa2CO3�������KΪNaHCO3��С�մ�Na2CO3������CaCO3��Ϊ��ɫ�������ɼ���CaCl2��Һ���𣬳��ְ�ɫ������ΪNa2CO3��
�ʴ�Ϊ��ȡI��K��ϡ��Һ���ֱ�μ�CaCl2��Һ�����ְ�ɫ������ΪI��
��������������������ʵ�ʣ��Խ����ѧʵ��������˼·�������ʣ�ʹ�����龳��ʵ��������ֻ�ѧ��STSE�Ĺ�ϵ������ѧ�Ա�ɫ��������ѧ��������ϵʵ�ʣ�ѧ�����õ�ѧϰ�ۣ����⿼���֪ʶ��࣬Ҳ�dz���˼ά�����ϴ���Ŀ�Ի�ѧ֪ʶ�������Ӧ��֪ʶ������������������й������Ǩ����������Ҫ��ϸߣ�����ʱע����ջ�ѧ��Ӧ��ѭ�����غ㶨�ɣ��Դ���д�йصĻ�ѧ����ʽ��

��ϰ��ϵ�д�
�����Ŀ