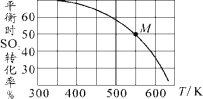
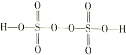
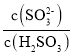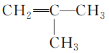��Ŀ����
����Ŀ��Cu3N�������õĵ�ѧ��ѧ�����ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ���̵������У������Ź㷺�ġ���������ľ����á�
��1��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ__��
��2����N3-������ͬ����������ԭ�����Ŀռ乹����__��
��3��Cu+�ĵ����Ų�ʽΪ__������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2O��__��
��4����Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��__����ȩ����H-C-O�ļ���_(����������������������С����)�Ҵ������е�H-C-O�ļ��ǡ�
��5��[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ���Ի���Cu(H2O)2Cl2���м��Եķ��ӵĽṹʽ��___��
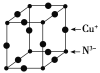
��6��Cu3N�ľ����ṹ��ͼ��ʾ��N3-����λ��Ϊ__��Cu+�İ뾶Ϊapm��N3-�İ뾶Ϊbpm��Cu3N���ܶ�Ϊ__g��cm-3(�����ӵ�������NA��ʾ)��
��7����(N2H4)�ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���������ȼ�ϵ����һ�ּ���ȼ�ϵ�أ���������Ⱦ���������Һ��20%��30%��KOH��Һ����������ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��__��
��8���������۷ɴ��ij����������(N2H4����̬)Ϊȼ�ϣ�Ϊ�������(N2H4)ȼ�չ������ͷŵ�����������NO2������������O2�������߷�Ӧ����N2��ˮ������
��֪����N2(g)+2O2(g)=2NO2(g) ��H1=+67.7kJ��mol-1����N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H2=-534kJ��mol-1��д���º�NO2��ȫ��Ӧ���Ȼ�ѧ����ʽ��__��
���𰸡�N>O>C V�� 1s22s22p63s23p63d10(��[Ar]3d10) Cu+��3d����ϵ���ȫ��������ṹ�ȶ� sp3��sp2 ���� 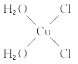 6
6 ![]() g/cm3 N2H4-4e-+4OH-=N2+4H2O 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=-1135.7kJ��mol-1
g/cm3 N2H4-4e-+4OH-=N2+4H2O 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=-1135.7kJ��mol-1
��������
(1)ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ������ȶ�״̬���������ͣ�ʧȥ��һ��������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N>O>C��
(2)��N3-������ͬ����������Ϊ�ȵ����壬��NO2-���ȵ�����ṹ���ƣ��������������Nԭ�Ӽ۲���ӶԸ���=2+1/2(5+1-2��2)=3�Һ���һ���µ��Ӷԣ�����ΪV�νṹ��
(3)Cu+�ĺ�����28�����ӣ����ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10��ԭ�ӹ������ȫ�ա��������ȫ����ʱ���ȶ���Cu+��3d�����ȫ�������ȶ���
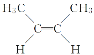
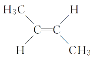
(4)��ȩ�����м���̼ԭ�Ӻ���4��������ȩ���ϵ�̼ԭ�Ӻ���3�����������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu(H2O)2(Cl)2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�����ṹʽΪ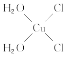 ��
��
(6)Cu3N�ľ����ṹ��ͼ���������=12��![]() =3��С�����=
=3��С�����=![]() ��8=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6�����������=[(2a+2b)��10-10cm]3��Cu3N���ܶ�=
��8=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6�����������=[(2a+2b)��10-10cm]3��Cu3N���ܶ�=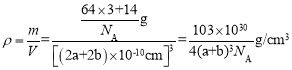 ��
��
(7)ԭ����и���ʧ���ӷ���������Ӧ�������Ŀ��Ϣ��֪��������ȼ�ϵ�طŵ�ʱ�±��������ɵ��������Ը�����Ӧ����ʽΪ��N2H4-4e-+4OH-=N2+4H2O��
(8)�º�NO2��ȫ��Ӧ�ķ���ʽΪ��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g)����֪����N2(g)+2O2(g)=2NO2(g) ��H1=+67.7kJ��mol-1����N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H2=-534kJ��mol-1����Ӧ����2-�ٿɵ��º�NO2��ȫ��Ӧ�ķ���ʽ�����ݸ�˹���ɸ÷�Ӧ��H=-534kJ��mol-1��2-67.7kJ��mol-1=-1135.7kJ��mol-1�������º�NO2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=-1135.7kJmol-1��

����Ŀ�������ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±���ʾ��
Ԫ�� | �ṹ�����ʵ���Ϣ |
A | �Ƕ�����Ԫ�أ�ϡ������Ԫ�س��⣩��ԭ�Ӱ뾶����Ԫ�أ�����Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
B | ��Aͬ���ڣ�������������Ӧ��ˮ��������� |
C | ����̬�⻯�K������ˮ��Һ̬ʱ����������� |
D | �Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�ɱ�������� |
E | Ԫ��ԭ�ӵ�L���Ӳ�����2�ԳɶԵ��� |
����ݱ�����Ϣ�ش��������⡣
��1��AԪ��ԭ�ӵĺ�������Ų�ʽΪ___��
��2��BԪ����Ԫ�����ڱ��е�λ��Ϊ___�����Ӱ뾶��B___�������������A��
��3��CԪ��ԭ�ӵĹ����ʾʽΪ___����ԭ�Ӻ�����___��δ�ɶԵ��ӣ�������ߵĵ����Ų��ڹ���ϣ��ù����___�Ρ�
��4��DԪ��ԭ�ӵĺ�������Ų�ʽΪ___��D-�Ľṹʾ��ͼΪ___��
��5��C��EԪ�صĵ�һ�����ܵĴ�С��ϵ��___����Ԫ�ط��ű�ʾ����
��6����֪CD3������DԪ����+1�ۣ��������£�C��DԪ�صĵ縺�Դ�С��ϵ��CD3___����Ԫ�ط��ű�ʾ����CD3��ˮ��Ӧ��IJ�����___���ѧʽ����