��Ŀ����
����Ŀ����һ���¶��£��������ˮϡ�����У���Һ�ĵ�������I�����ˮ�����V�仯��������ͼ��ʾ����ش�
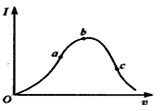
��1����O���㵼������ΪO�������� ��
��2��a��b��c������Һ��c��H+����С�����˳��Ϊ ��
��3��a��b��c�����д������������� ��ˮ����̶������� ��
��4����ʹc����Һ��c��CH3COO-����ߣ������´�ʩ�пɲ�ȡ ����������
A������
B��������
C���ӱ�����
D���������KOH
E����ˮ
F���ӹ���CH3COONa
G����Zn��
���𰸡���1���������Է�����ʽ���ڣ������룬�������ƶ������������Բ�����
��2�� c<a<b ��3�� c c ��4�� ACDFG
��������
�����������1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ����������û�������ƶ������ӣ����Ա�������磻
��2����������Խǿ������Ũ��Խ��������Ũ��Խ��pHԽС����a��b��c������Һ��pHΪb��a��c��������Ũ����С�����˳��Ϊcab��
��3����ҺԽϡ��Խ�ٽ�������룬CH3COOH�ĵ���̶�������c����Һ��������Ũ��ԽС����ˮ�ĵ������Ƴ̶�ԽС��c��������Ũ����С��ˮ�ĵ���̶����
��4��Ҫʹ���������Ũ�������Բ��ü��ȡ����뺬�д�������ӵ����ʡ�����������ӷ�Ӧ�����ʣ�A�����ȴٽ�������룬����Һ�д��������Ũ������A��ȷ��B�����������ƴ�����룬B����C���ӱ����ᣬ�����Ũ������C��ȷ��D����NaOH���壬�������ƺ������ӷ�Ӧ�ٽ�������룬���Դ��������Ũ������D��ȷ��E����ˮϡ���ܴٽ�������룬�����������Ũ�ȼ�С��E����F���ӹ���CH3COONa�������ƴ�����룬�������Ƶ�����Ĵ�������Ӵ������ƴ��������Ĵ�������ӣ����Դ��������Ũ������F��ȷ��G������п�����������ӷ�Ӧ���ٽ�������룬���Դ��������Ũ������G��ȷ����ѡACDFG��

����Ŀ����a��b��c��d�ĸ������缫���йصĻ�ѧװ�á����ַ�Ӧ�������£�
ʵ��װ�� |
|
|
|
|
ʵ������ | a��������С�� b���������� | b�������������c���ޱ仯 | d���ܽ⣬c����������� | ������a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳����
A. a>b>c>d B. b>c>d>a C. a>b>d>c D. d>a>b>c