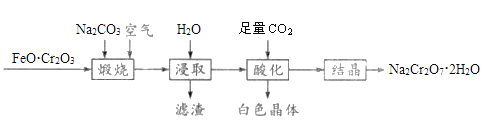
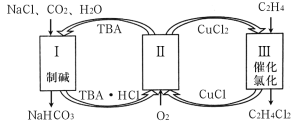
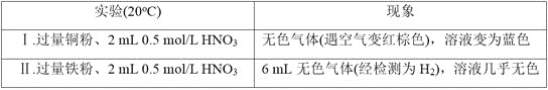
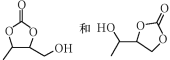��Ŀ����
����Ŀ�������������ƶ�����Ҫ�Ĺ�ҵ��Ʒ����ش�
(1)��ҵұ�����Ļ�ѧ����ʽ��___________________________________________��
(2)���������ӽ���Ĥ����ⱥ��ʳ��ˮ��NaOH���乤��ԭ��������ͼ��ʾ��
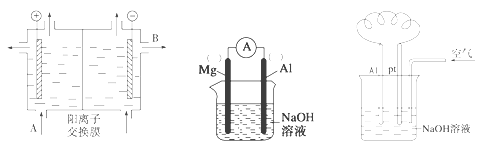
����д��A��B�������ʵ����ƻ�ѧʽ��A___________________��B____________________
����д�����ʳ��ˮ�����ӷ���ʽ__________________________________________
(3)��þ������ƬΪ�缫����NaOH��ҺΪ�������Һ��Ƶ�ԭ���������ͼ��
����������Ϊ________(��Mg��Al)��
����ԭ��ص��ܷ�ӦʽΪ____________________________________________
(4)��������ȼ�ϵ�ؿ����ڵ綯������ͨ����NaOH��ҺΪ���Һ�����Ͻ�Ϊ������ͨ������ļ�Ϊ����(������ͼ)����
�����ĵ缫��ӦʽΪ__________________________________��
�����ĵ缫��ӦʽΪ___________________________________��
���𰸡�2Al2O3![]() 4Al +3O2 �� ŨNaCl ŨNaOH 2NaCl+2H2O=���=2NaOH+H2��+Cl2�� Al 2Al+2NaOH+2H2O=2NaAlO2+3H2�� 2Al-6e-+6OH-=Al2O3+3H2O O2+4e-+2H2O=4OH-
4Al +3O2 �� ŨNaCl ŨNaOH 2NaCl+2H2O=���=2NaOH+H2��+Cl2�� Al 2Al+2NaOH+2H2O=2NaAlO2+3H2�� 2Al-6e-+6OH-=Al2O3+3H2O O2+4e-+2H2O=4OH-
��������
��1����ҵұ����Ϊ������ڵ�Al2O3������ʽΪ2Al2O3![]() 4Al +3O2 ����
4Al +3O2 ����
��2�������ӽ��������NaCl������ʧ���ӵõ�Cl2�������õ��ӵõ�H2������ͼ���Է�������ŨNaClͨ��A�࣬ϡNaOHͨ��B�࣬���Եõ�ŨNaOH����Aͨ�����ŨNaCl��B��������ŨNaOH�����ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O=���=2NaOH+H2��+Cl2����
��3����þ������ƬΪ�缫����NaOH��ҺΪ�������Һ��Ƶ�ԭ��أ���ΪAl���Ժ�NaOH������Ӧ����AlΪ������MgΪ�������缫�ܷ�ӦΪ2Al+2NaOH+2H2O=2NaAlO2+3H2��
��4����������ȼ�ϵ�ؿ����ڵ綯������ͨ����NaOH��ҺΪ���Һ�����Ͻ�Ϊ������ͨ������ļ�Ϊ�������ܷ�ӦΪ4Al+3O2=2Al2O3��������ӦΪ2Al-6e-+6OH-=Al2O3+3H2O����������ʽΪO2+4e-+2H2O=4OH-��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�