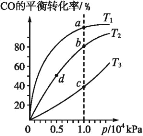
��Ŀ����
����Ŀ��ijʵ�����С���ͬѧ��ʵ�����������Ʊ�������������Һ�Ʊ����������![]() ����ΪĪ����
����ΪĪ����![]() ��
��![]() ��
��![]() ��Է�������Ϊ
��Է�������Ϊ![]() ���þ����һ���������ȶ���������ˮ���������Ҵ���
���þ����һ���������ȶ���������ˮ���������Ҵ���
(һ)�Ʊ�����������Һ![]() ʵ�鲽������
ʵ�鲽������![]()
(1)����٣�����ϡ�����Ŀ����________��
(2)����ڣ����뻹ԭ��м��۲쵽��������________��
ʵ��ǰ�����ü���Һϴ����м����Ŀ����________��
(��)�Ʊ�Ī����![]() ���������
���������![]()
(3)��![]() ��Һ�м����Թ���������隣�����Һ������Һ����ȡ
��Һ�м����Թ���������隣�����Һ������Һ����ȡ![]() �ľ��������________�����Ҵ�ϴ�ӡ����
�ľ��������________�����Ҵ�ϴ�ӡ����
(��)����Ī���μ���ʱ�ķֽ����
Ī�������ȷֽ⣬��ͬѧ��Ϊ�ֽ������������¼��������
![]() ��
��![]() ��
��![]() ��
��![]()
![]() ��
��![]() ��
��![]() ��
��![]()
![]() ��
��![]() ��
��![]() ��
��![]()
![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
(4)�������������������ȷ������________![]() �����
�����![]() ��������
��������
(5)��ͬѧ��ΪĪ���ηֽ�IJ����п��ܺ���![]() ��
��![]() ��
��![]() ��Ϊ��֤����Ĵ��ڣ���ͬѧ������װ�ý���ʵ�飺
��Ϊ��֤����Ĵ��ڣ���ͬѧ������װ�ý���ʵ�飺
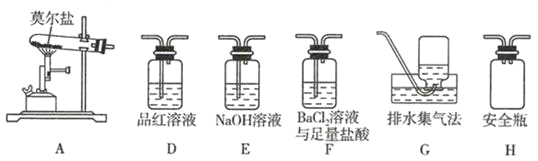
����ͬѧ��ʵ���У�װ���������ӵĺ���˳��Ϊ![]() ________
________![]() ��
��
��֤���ֽ�����к���![]() ��ʵ��������________��
��ʵ��������________��
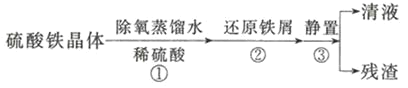
(��)�ⶨʵ��![]() ��
��![]() ���þ�����
���þ�����![]() �Ĵ��ȳ�ȡ
�Ĵ��ȳ�ȡ![]() �����������Ʒ���Ƴ�
�����������Ʒ���Ƴ�![]() ��Һ��ȡ
��Һ��ȡ![]() ��Ʒ��Һ����ʵ�飬װ����ͼ��ʾ��
��Ʒ��Һ����ʵ�飬װ����ͼ��ʾ��
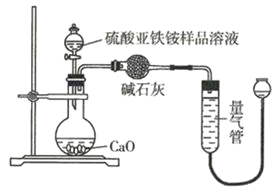
(6)��ʵ����![]() �����Ϊ
�����Ϊ![]() ������Ϊ��״����
������Ϊ��״����![]() ����������������Ʒ�Ĵ���Ϊ________
����������������Ʒ�Ĵ���Ϊ________![]() �г�����ʽ����
�г�����ʽ����![]() ��
��
���𰸡�����![]() ��ˮ�� �������ܽ⣬��Һ�ɻ�ɫ��Ϊdz��ɫ������ɫ�������� ��ȥ��м��������� ����Ũ������ȴ�ᾧ������
��ˮ�� �������ܽ⣬��Һ�ɻ�ɫ��Ϊdz��ɫ������ɫ�������� ��ȥ��м��������� ����Ũ������ȴ�ᾧ������ ![]()
![]()
![]() �г��ְ�ɫ����
�г��ְ�ɫ���� ![]()
��������
(1)����٣�����ϡ�����Ŀ��������Fe3+��ˮ�⣬
�ʴ�Ϊ������Fe3+��ˮ�⣻
(2)����ڣ����뻹ԭ��м����м���ἰFe3+��Ӧ���۲쵽���������������ܽ⣬��Һ�ɻ�ɫ��Ϊdz��ɫ������ɫ�������ɣ�����Һ���Գ�ȥ��м��������ۣ�
�ʴ�Ϊ���������ܽ⣬��Һ�ɻ�ɫ��Ϊdz��ɫ������ɫ�������� ����ȥ��м��������ۣ�
(3)ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�������Դ���Һ�л����������泥�
�ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˣ�
(4)a.����ֽ������Fe2O3��SO3��NH3��H2O�������ʣ���ֻ��FeԪ�صĻ��ϼ����ߣ�û�л��ϼ۽��͵�Ԫ�أ��Dz����ܵģ�
c.����ֽ������FeO��SO2��NH3��H2O�������ʣ���ֻ��SԪ�صĻ��ϼ۽��ͣ�û�л��ϼ����ߵ�Ԫ�أ��Dz����ܵģ��ʲ���a��c��������
��ѡ��ac��
(5)����ͬѧ��ΪĪ���ηֽ�IJ����п��ܺ���SO3��g����SO2��g����N2��g������BaCl2����������Ļ��Һ����SO3��g������Ʒ����Һ����SO2��g����N2������ˮ������ˮ��,�ռ�������SO3�ܱ�ˮ��Һ����,����Ӧ�ȼ���SO3��g�����ټ���SO2��g������NaOH��Һ��ȥSO2������ˮ���ռ�N2����װ�õĺ�������˳��ΪA��H��F��D��E��G����BaCl2����������Ļ��Һ����SO3��g��ʱ������Ϊ��Һ�г��ְ�ɫ������
�ʴ�Ϊ��F��D��E��![]() �г��ְ�ɫ������
�г��ְ�ɫ������
(6)��״����VL���������ʵ���Ϊ![]() =
=![]() mol��mg�����������Ʒ��NԪ�ص����ʵ���Ϊ
mol��mg�����������Ʒ��NԪ�ص����ʵ���Ϊ![]()
![]() mol=
mol=![]() mol����������������Ʒ�Ĵ���2��
mol����������������Ʒ�Ĵ���2��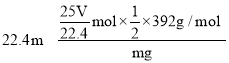 ��100%=
��100%=![]() ��100%
��100%
�ʴ�Ϊ��![]() ��100%��
��100%��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�