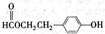��Ŀ����
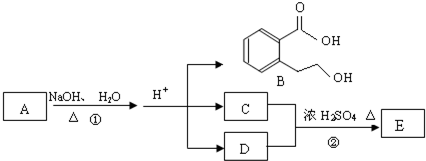
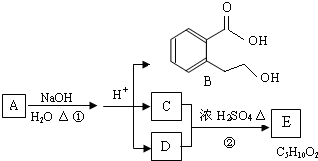
A��B��C��D��E��Ϊ�л����������֮��Ĺ�ϵ������ʾ(��ʾ��RCH===CHR����KMnO4������Һ�з�Ӧ����RCOOH��R��COOH������R��R��Ϊ���)
�ش��������⣺
(1)ֱ��������A����Է�������С��90��A������̼����Ԫ�ص�����������Ϊ0.814������Ϊ��Ԫ�أ���A�ķ���ʽΪ________��
(2)��֪B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������Ũ����Ĵ��£�B��������C2H5OH������Ӧ�Ļ�ѧ����ʽ��__________________________����Ӧ����Ϊ________��
(3)A��������������÷ų���������ʹ���CCl4��Һ��ɫ����A�Ľṹ��ʽ��____________________��
(4)D��ͬ���칹���У�����NaHCO3��Һ��Ӧ�ų�CO2����________�֣�����Ӧ�Ľṹ��ʽΪ________��
(1)C5H10O
(2)HOOCCH2COOH��2C2H5OH  2H2O��C2H5OOCCH2COOC2H5��������Ӧ(��ȡ����Ӧ)
2H2O��C2H5OOCCH2COOC2H5��������Ӧ(��ȡ����Ӧ)
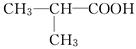
(3)CH3CH===CHCH2CH2OH (4)2��CH3CH2CH2COOH��(CH3)2CHCOOH
��������
����������������Ϣ��֪B��CΪ����COOH�����ʣ���C��D��ת����֪CΪCH3COOH����A�бغ�CH3CH===CH���ṹ����(3)��A��Na��������Ȼ�̼��Һ��Ӧ�����ƶ�AΪϩ���࣬��(2)������֪BΪ��Ԫ���ᣬ���AΪϩ������ȷ��AΪ��CH3CH===CH���͡�CH2��OH�ṹ�����ʣ���(1)��֪������������Ϊ1��0.814��0.186����16��0.186��86��90����֪A�н���1����ԭ�ӣ������A�ķ���ʽΪCnH2nO��ͨ����Է�������Ϊ86��֪����ʽΪC5H10O������ΪA��ֱ���������ṹ��ʽΪCH3CH===CHCH2CH2OH.��һ����֪BΪHOOCCH2COOH��B��C2H5OH���Է���������Ӧ������ʽΪC4H8O2��ͬ���칹��������NaHCO3��Ӧ��Ϊ���ᣬΪC3H7COOH������2��ͬ���칹�壺CH3��CH2��CH2��COOH��
 ��
��
���㣺�����л���ṹ��ʽ���л���Ӧ���͡�ͬ���칹���Լ���ѧ����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������֪ʶ�Ĺ�����ѵ����ͬʱ��˶�ѧ�������������ͽ��ⷽ����ָ��ѵ��������Ĺؼ������ճ��������ŵĽṹ�����ʣ��ر��ǹ�����֮����ת����ϵ������������ѧ���������������淶�Ĵ���������