��Ŀ����
ij�о���ѧϰС��̽�����л�ѧ��Ӧ��������
���������ữ�ĸ��������Һ����KI��KMnO4+KI+H2SO4��MnSO4+I2+KIO3+K2SO4+H2O��
����Cu2S��һ��Ũ�ȵ�HNO3��H2SO4���Һ��Ӧ��
Cu2S+HNO3+H2SO4��Cu��NO3��2+CuSO4+NO2+NO+H2O��
��������ͭ�ڸ����·ֽ⣺CuSO4��CuO+SO3+SO2+O2��
����������������������Һ��Ӧ��NaOH+H2S��NaHS+Na2S+H2O��
��1�����������ѧ��Ӧ�����ʵĻ�ѧ������֮���ǹ̶��ġ����ǣ�������ѧ��Ӧ�Ļ�ѧ�������Dz�ȷ���ģ������ʻ�ѧ�������ж��顣��������ʵ����������ɻ�ѧ�������ж���Ļ�ѧ��Ӧ���ص㣺_______��
��2����Ӧ������ֻ��ȷ��_______�����ʵ���֮�ȣ���ѧ����ʽ�Ļ�ѧ������Ҳ��֮ȷ����
��3��������Ϊ��Ӧ������������Ӧʽ�ӺϵĽ���������������������ѧ��Ӧ�Ӻ϶��ɵ�_____���ڷ�Ӧ�����и��ݻ��ϼ��غ㣬_______�����ʵ���֮���ǹ̶��ģ����������غ㣬_______�����ʵ���֮���ǹ̶��ġ�
��4�������⻯�ơ����ƵĻ�ѧ�������ֱ�Ϊa��b������a��b��ʾ�û�ѧ��Ӧ�Ļ�ѧ�������Ļ�ѧ����ʽΪ________��
��1����һ���������ͬ��Ԫ�ص����������ԭ���������ּ��������ϣ� �ڶ��������һ����Ӧ������Ϊ������Ӧ�ļӺϣ����ಽ��Ӧ�ļӺϷ�Ӧʽ�����ж��黯ѧ������
��2��NO��NO2
��3��CuSO4��CuO+SO2+O2��CuSO4��CuO+SO3�� SO2��O2�� CuSO4��CuO
��4����a+2b��NaOH+��a+b��H2S=aNaHS+bNa2S+��a+2b��H2O
�����������
��������1����Ӧ���IJ�����I2��KIO3����������������е�Ԫ�أ����ǵı������������Է�Ӧ�и����ʵĻ�ѧ��������ȷ������Ӧ���IJ����л�ԭ������NO��NO2�������е�Ԫ�أ���Ӧ�����Էֳ�������ԭ��Ӧ���������ԭ��Ӧ�ļӺϣ�CuSO4��CuO+SO2+O2��CuSO4��CuO+SO3�Ӻϵõ���CuSO4��CuO+SO3+SO2+O2����Ӧ������������Ϊ�ֲ���Ӧ�Ҷ�Ϊ��������ԭ��Ӧ��NaOH+H2S��Na2S+H2O��NaOH+H2S��NaHS+H2O�ļӺϡ������������������Թ���һ����ɡ�
��2��Cu2S+HNO3+H2SO4��Cu��NO3��2+CuSO4+NO2+NO+H2O�У���ΪNO��NO2�����ʵ����������õ�����������ȷ��������ֻҪȷ��NO��NO2�����ʵ���֮�Ȼ�ͬ���������֮�ȣ���ѧ�������̶��ˣ���NO��NO2���ʵ���֮��Ϊ1��1ʱ����ƽ������ѧ����ʽ��2Cu2S+14HNO3=2Cu��NO3��2+2CuSO4+5��NO+NO2��+7H2O��
��3�����ݻ��ϼ��غ㣬��ʧȥ���ӵ�����������õ����ӵ�����������1molSO2�õ�2mole-������1molO2ʧȥ4mole-����n��SO2����n��O2��=2��1�����������غ㶨�ɣ�n��CuSO4��=n��CuO������4���������ƺ����⻯�ƵĻ�ѧ������������ԭ���غ���ƽ�÷�Ӧ�������Ļ�ѧ����ʽ����a+2b��NaOH+��a+b��H2S=aNaHS+bNa2S+��a+2b��H2O��

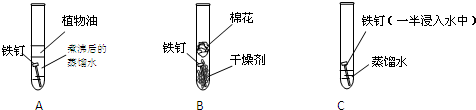
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д� Fe2++Ag+����ش��������⣺
Fe2++Ag+����ش��������⣺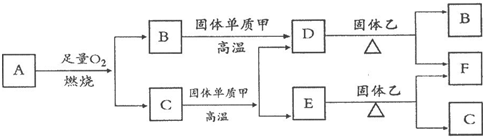
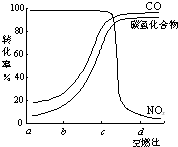
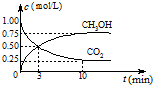 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����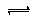 CO+3H2
CO+3H2