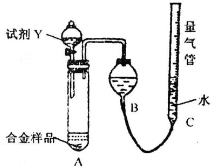
��Ŀ����
��2���¶ȼƵ�������_____________________�����Ƭ��������______________________��
��3��д��ʵ���в�����ϩ�Ļ�ѧ����ʽ��_______________________________��
��4����ͬѧ��Ϊ����ˮ��ɫ��������֤����ϩ���в������ԣ���ԭ������ƿ��Һ����غ�ɫ������
__________���塣��ͬѧ������ϸ�۲����Ϊ���Թ�����һ�������֤����ϩ���в������ԣ����������_______________����ͬѧΪ��֤��һ��Ӧ�Ǽӳɶ�����ȡ��������˽������������պ���pH��ֽ�����Է�Ӧ����Һ�����ԣ�������____________________________________��
��5����������ʵ������ƿ�ڷ�Һ����ȷ������_______________��
A����Һֱ�ӵ�����ˮ��
B����Һ����շ�Һ����
C����ˮ������ƿ��
D����Һ����ʢ��ˮ������Ͱ�У����������ٵ�����ˮ��
��2�����Ʒ�Ӧ�¶���170�棻��ֹ����
��3��
��4��O2���в�����ˮ����״�����ɣ���������ȡ����Ӧ���ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤
��5��D

��A������ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����
![]()
ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
1 | 10 mL FeSO4��Һ | 10 mL NH3 | ���ɰ�ɫ���������ɫ |
2 | 20 mL H2S | 10 mL SO2 |
|
3 | 30 mL NO2����Ҫ�� | 10 mL H2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
4 | 15 mL Cl2 | 40 mL NH3 |
|
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ___________ɫ��д��������ɫ�Ļ�ѧ����ʽ_______________��
��2��ʵ��2����Ͳ�ڵ������ǣ���________���ɣ�����___________�ƶ�������⡱�����ڡ�����������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��__________��Һ�С�
��3��ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������__________��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________________��
��4��ʵ��4�У���֪��3Cl2+2NH3![]() N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
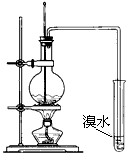
��B��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ
_____________________________________________________________________
_____________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
��2����������ˮԡ���ò���ͬ��
��������____________________���ҵ�������_____________________��
��3����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������____________________________������ƿ���ռ������������Ҫ�ɷ���______________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���______________����д��ĸ����
a.�Ȼ�����Һ b.��
c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��_____________����ʵ��������ƣ����ɳ�ȥ��
�״���Դ�ḻ���۸�������������淽�㣬��һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ����
��1����ҵ�����״��ij��÷����ǣ�CO(g)+2H2(g) ![]() CH3OH(g) ��H = ��90.8kJ/mol�����ں��º��ݵ������ڽ��з�ӦCO(g)+2H2(g)
CH3OH(g) ��H = ��90.8kJ/mol�����ں��º��ݵ������ڽ��з�ӦCO(g)+2H2(g) ![]() CH3OH(g)�����б�ʾ�÷�Ӧ�ﵽƽ��״̬�ı�־�� ������ĸ��ţ���
CH3OH(g)�����б�ʾ�÷�Ӧ�ﵽƽ��״̬�ı�־�� ������ĸ��ţ���
A�������л��������ܶȲ��仯
B��CO�ٷֺ������ֲ���
C�������л�������ѹǿ���仯
D����1��H��H�����ɵ�ͬʱ�� 3��C��H������
��2���Ƽ״�����Ҫ��H2���������з�Ӧ��ȡ��H2O(g)+CO(g) ![]() H2(g)+ CO2(g)
H2(g)+ CO2(g)
��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K = 1������ʼʱc(CO)=1mol•L-1��c(H2O)=2mol•L��1���Իش��������⣺
�ٸ��¶��£���Ӧ����һ��ʱ����H2��Ũ��Ϊ0.5mol•L-1�����ʱ�÷�Ӧ
v(��) v(��)�����������������������
������Ӧ�¶Ȳ��䣬�ﵽƽ���H2O��ת����Ϊ ��
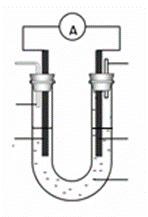
��3��ijʵ��С�����������ͼ��ʾ�ļ״�ȼ�ϵ��װ�á�
| ||||||||||
 | ||||||||||
| ||||||||||
| ||||||||||
�ٸõ�ع���ʱ��OH�� �� ���ƶ����a����b������
�ڹ���һ��ʱ������Һ��pH��С���õ�ظ����缫��ӦʽΪ ��