��Ŀ����
��2013?������һģ����þ�Ͻ����ִ����졢������������ҵ����Ҫ���ϣ�Ϊ�ⶨij��þ�Ͻ𣨲�������Ԫ�أ���þ������������ijʵ��С�����������ʵ�鷽����ÿ����������ȡ5.4g��ĩ״��Ʒ����̽�����밴Ҫ��ش��������⣺
[ʵ��l]��þ�Ͻ�
�ⶨʣ�����������
��֪���Լ�X��ɫ��ӦΪ��ɫ��
��1���������õ���Ʒ����V1mL 2.0mol/L�Լ�X�У���ַ�Ӧ���˷�Ӧ�����ӷ���ʽΪ
��2�������ˡ�����������壬���þ����������
[ʵ��2]��þ�Ͻ�
�ⶨ����
��l��������þ��������������ʵ�黹��ⶨ��������
��2�����ÿ�������O2����ʵ�飬��ⶨþ������������
Mg3N2]
[ʵ��3]��þ�Ͻ�
�ⶨ������ɫ��������
ʵ��װ������ͼ��
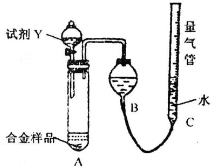
��1����ʵ����ʢװҩƷ֮ǰӦ���Ƚ��е�ʵ�鲽����
��2��ͬѧ��ѡ���Լ�Y����ʵ�飬������װ��A����ƷѸ���ܽ���ȫ����������������C��Һ����B��Һ����ƽ����ò����������ΪV2L[������Ϊ��״���£�A��Һ��������Բ��ƣ�������3����ͬ]���Լ�Y��������
��3��ͬѧ�ҽ��Լ�Y��Ϊʵ��1�е��Լ�X�������������ȷ����ַ�Ӧ���ò����������ΪV3L����þ����������Ϊ
��100%
��100%����V3�ı���ʽ����
[ʵ��l]��þ�Ͻ�
| �����Լ�X |
��֪���Լ�X��ɫ��ӦΪ��ɫ��
��1���������õ���Ʒ����V1mL 2.0mol/L�Լ�X�У���ַ�Ӧ���˷�Ӧ�����ӷ���ʽΪ
2Al+2OH-+2H2O�T2AlO2-+3H2��
2Al+2OH-+2H2O�T2AlO2-+3H2��
��V1��ȡֵ��Χ����100mL
��100mL
mL����2�������ˡ�����������壬���þ����������
ƫ��
ƫ��
���ƫ�ߡ�����Ӱ�족��ƫ�͡�������ԭ�������˺�û��ϴ��
���˺�û��ϴ��
��[ʵ��2]��þ�Ͻ�
| ����O2������� |
| �ܷ�(�����������ƶ��Ļ���) |
��l��������þ��������������ʵ�黹��ⶨ��������
���պ���������
���պ���������
����2�����ÿ�������O2����ʵ�飬��ⶨþ������������
ƫ��
ƫ��
�ƫ�ߡ�����Ӱ�족��ƫ�͡�����[��֪��3Mg+N2
| ||
[ʵ��3]��þ�Ͻ�
| �����Լ�Y |
ʵ��װ������ͼ��

��1����ʵ����ʢװҩƷ֮ǰӦ���Ƚ��е�ʵ�鲽����
���װ�õ�������
���װ�õ�������
����2��ͬѧ��ѡ���Լ�Y����ʵ�飬������װ��A����ƷѸ���ܽ���ȫ����������������C��Һ����B��Һ����ƽ����ò����������ΪV2L[������Ϊ��״���£�A��Һ��������Բ��ƣ�������3����ͬ]���Լ�Y��������
ϡ�����ϡ����
ϡ�����ϡ����
������þ����������ƫ�͵����������ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ��
���ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ��
����3��ͬѧ�ҽ��Լ�Y��Ϊʵ��1�е��Լ�X�������������ȷ����ַ�Ӧ���ò����������ΪV3L����þ����������Ϊ
| 1-V3 |
| 6.72 |
| 1-V3 |
| 6.72 |
������[ʵ��l]��1���Լ�X��ɫ��ӦΪ��ɫ��ӦΪNaOH������2Al+2NaOH+2H2O�T2NaAlO2+3H2����þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
[ʵ��2]��1��Mg��Al����������Ӧ��
��2�����ÿ�������O2����ʵ�飬����3Mg+N2
Mg3N2��2Mg+CO2
2MgO+C���ⶨ���ɹ�����������
[ʵ��3]��1���ⶨ����������Ӧ�ȼ��װ�õ������ԣ�
��2������������ϡ��ķ�Ӧ����������Al�ĺ���Խ����������Խ�ࣻ
��3���Լ�Y��Ϊʵ��1�е��Լ�X������2Al+2NaOH+H2O�T2NaAlO2+3H2�������Al�ĺ������ټ���Mg������������
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
[ʵ��2]��1��Mg��Al����������Ӧ��
��2�����ÿ�������O2����ʵ�飬����3Mg+N2
| ||
| ||
[ʵ��3]��1���ⶨ����������Ӧ�ȼ��װ�õ������ԣ�
��2������������ϡ��ķ�Ӧ����������Al�ĺ���Խ����������Խ�ࣻ
��3���Լ�Y��Ϊʵ��1�е��Լ�X������2Al+2NaOH+H2O�T2NaAlO2+3H2�������Al�ĺ������ټ���Mg������������
����⣺[ʵ��l]��1���Լ�X��ɫ��ӦΪ��ɫ��ӦΪNaOH������2Al+2NaOH+2H2O�T2NaAlO2+3H2���������ӷ�ӦΪ2Al+2OH-+2H2O�T2AlO2-+3H2��������5.4g��ΪAl��n��Al��=
=0.2mol����Ҫ����������Ϊ0.2mol�������Ϊ
=0.1L=100mL��ʵ����Ҫ����������Һ�����V1��100mL��
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2������100mL��
��2����û��ϴ�ӣ���þ�ϻḽ��ƫ�����Ƶ����ʣ���δϴ�ӵ��²ⶨ��þ������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ����˺�û��ϴ�ӣ�
[ʵ��2]��1��Mg��Al����������Ӧ�����ɽ������������ⶨ��������������ʴ�Ϊ�����պ�����������
��2�����ÿ�������O2����ʵ�飬����3Mg+N2
Mg3N2��2Mg+CO2
2MgO+C���ⶨ���ɹ�������������þ����������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
[ʵ��3]��1���ⶨ����������Ӧ�ȼ��װ�õ������ԣ����Ը�ʵ����ʢװҩƷ֮ǰӦ���Ƚ��е�ʵ�鲽��Ϊ���װ�õ������ԣ��ʴ�Ϊ�����װ�õ������ԣ�
��2���������ϡ�����ϡ����ķ�Ӧ������������ӦΪ���ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ�ͣ�
�ʴ�Ϊ��ϡ�����ϡ������ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ�ͣ�
��3���Լ�Y��Ϊʵ��1�е��Լ�X����2Al+2NaOH+H2O�T2NaAlO2+3H2����֪��n��H2��=
mol����n��Al��=
mol��Al�ĺ���Ϊ
g������Mg����������Ϊ
��100%=
��100%���ʴ�Ϊ��
��100%��
| 5.4g |
| 27g/mol |
| 0.2mol |
| 2.0mol/L |
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2������100mL��
��2����û��ϴ�ӣ���þ�ϻḽ��ƫ�����Ƶ����ʣ���δϴ�ӵ��²ⶨ��þ������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ����˺�û��ϴ�ӣ�
[ʵ��2]��1��Mg��Al����������Ӧ�����ɽ������������ⶨ��������������ʴ�Ϊ�����պ�����������
��2�����ÿ�������O2����ʵ�飬����3Mg+N2
| ||
| ||
[ʵ��3]��1���ⶨ����������Ӧ�ȼ��װ�õ������ԣ����Ը�ʵ����ʢװҩƷ֮ǰӦ���Ƚ��е�ʵ�鲽��Ϊ���װ�õ������ԣ��ʴ�Ϊ�����װ�õ������ԣ�
��2���������ϡ�����ϡ����ķ�Ӧ������������ӦΪ���ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ�ͣ�
�ʴ�Ϊ��ϡ�����ϡ������ȷ�Ӧ���¶����ߣ��ⶨ�����������ƫ����Al�ĺ���Խ����������Խ�࣬�������������ʱ�����Mg����������ƫ�ͣ�
��3���Լ�Y��Ϊʵ��1�е��Լ�X����2Al+2NaOH+H2O�T2NaAlO2+3H2����֪��n��H2��=
| V3 |
| 22.4 |
| V3 |
| 33.6 |
| 27V3 |
| 33.6 |
5.4g-
| ||
| 5.4g |
| 1-V3 |
| 6.72 |
| 1-V3 |
| 6.72 |
���������⿼��ѧ������þ�ͽ������Ļ�ѧ�����Լ���ѧʵ�鷽�������֪ʶ��ע��ʵ��ԭ����ʵ���з����Ļ�ѧ��Ӧ����Ŀ�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�ע��ѧ��������˼ά��ѵ����

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ

