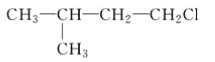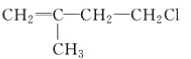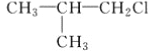��Ŀ����
����Ŀ����ȡ������ʵ�������²��裺
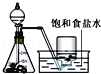
����һ��������������м���ֲ����8mL���Ҵ�8mL��NaOH��Һ4mL
���ڲ��Ͻ����£�����������Һ�����ȣ�ֱ���������
�ۼ������ȣ�ֱ��������Ӧ���
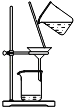
�ܰ�ʢ�����������������ˮԡ����ȴ���ȴ�Ƭ�̣��������м�20mL������ˮ���ٷ�����ˮ����ȴ��Ȼ�����25mL NaCl������Һ��ֽ���
����ɴ���˳��������ʣ���ȥ��Һ���ѹ������ʼ��ڡ�ѹ����״�����ɣ����÷���
����ʵ�飬��գ�
��1�����Ʒ���ʱ�����Ҵ����������Ҵ���ʲô���ʣ�_________��
��2�������֤������Ӧ����ɣ�_________��
��3���ڲ������м��뱥��NaCl��Һ��������_________��
��4��д��Ӳ֬�����������������Ӧ�Ļ�ѧ����ʽ_________��
��5��ȡ���������õ�������_________��
��6����ҵ�ϴ��ģ������ˮ�������ȵ��ŵ�_________����
���𰸡� ֲ���͡�NaOH��Һ�������Ҵ����ܣ������Ҵ���ʹֲ���ͺ�NaOH��Һ��ֽӴ��������ڷ�Ӧ�Ľ��� ȡ��ӦҺ��������ˮ�У���Һ�������͵Σ���˵��ˮ������� ������ʹ�������� 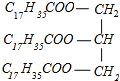 +3NaOH��3C17H35COONa+
+3NaOH��3C17H35COONa+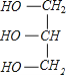 ����ǯ ���Ⱦ��ȣ��¶ȱ��ڿ��ƣ����ײ����ό����
����ǯ ���Ⱦ��ȣ��¶ȱ��ڿ��ƣ����ײ����ό����
��������(1)����ȡ����ʱ�����Ҵ�����Ϊ��ʹ����֬��ˮ���Ҵ��г�ֻ����
(2)�����ַ�ӦҺ������ˮ������Һ�������͵�����˵��ˮ���������
(3)���뱥���Ȼ�����Һ������֬�������ܹ��ӻ��Һ��������
(4)Ӳ֬��������ڼ��������·���������Ӧ,Ӳ֬����������������Ʒ�Ӧ���� C17H35COONa�������
(5)ʵ�������ȡ��������Ӧ��ʹ������ǯ��
(6)��������ˮ��������ʹ���Ⱦ������¶ȱ��ڿ���,���ײ����ό����������
(1)��Ϊֲ����������������Һ��������,�����ڷ�Ӧ�Ľ���,��ֲ���͡�NaOH��Һ�������Ҵ������������Ҵ���ʹֲ���ͺ�NaOH��Һ��ֽӴ��������ڷ�Ӧ�Ľ�������ȷ��: ֲ���͡�NaOH��Һ�������Ҵ����ܣ������Ҵ���ʹֲ���ͺ�NaOH��Һ��ֽӴ��������ڷ�Ӧ�Ľ�����
(2)��֬������ˮ,������֤������Ӧ����ɵķ���Ϊ��ȡ��ӦҺ,������ˮ��,��Һ�������͵�����˵��ˮ�����������ȷ����ȡ��ӦҺ��������ˮ�У���Һ�������͵Σ���˵��ˮ���������
(3)������Ӧ����������뱥��ʳ��ˮ���ܹ�ʹ���ɵĸ�֬�����Ʒ���������ʹ������������ȷ��:������ʹ����������
(4)Ӳ֬����������������Ʒ�Ӧ����C17H35COONa �����,��Ӧ����ʽΪ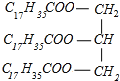 +3NaOH��3C17H35COONa+
+3NaOH��3C17H35COONa+ ����ȷ��:
����ȷ��: 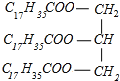 +3NaOH��3C17H35COONa+
+3NaOH��3C17H35COONa+ ��
��
(5)ʵ���������ȡ���������õ�����Ϊ����ǯ����ȷ��������ǯ��
(6)��ҵ�ϴ��ģ������ˮ��������,�ŵ�����ʹ��Ӧ���������Ⱦ���,�¶ȱ��ڿ���,Ҳ���ײ����ό��������ȷ�������Ⱦ������¶ȱ��ڿ��������ײ����ό������

����Ŀ������ʵ���Ӧ�Ľ�����ȷ����
ѡ�� | A | B | C | D |
װ�� |
|
|
|
|
���� | ��Ũ������������������� | ��֤���ǽ����� Cl��C��Si | �����Cl2��KI��Һ��Ӧ���ɵĵ� | ��ɫ����һ����BaSO4 |
A. A B. B C. C D. D