��Ŀ����
����Ŀ��̼���仯��������Դ�Ͳ��Ϸ�����й㷺����;���ش��������⣺
��1����֪CH4(g)+![]() O2(g)=CO(g)+2H2O(l)
O2(g)=CO(g)+2H2O(l) ![]() =-607.31kJ/mol
=-607.31kJ/mol
2CO(g)+ O2(g)=2CO2(g) ![]() =-566.0kJ/mol
=-566.0kJ/mol
д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ________________��
��2����Ȼ����һ����Ҫ��;����ȡH2����ԭ��Ϊ��CO2(g)��CH4(g) ![]() 2CO(g)��2H2(g) ��
2CO(g)��2H2(g) ��
���ܱ�������ͨ�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH4��CO2����һ�������·�����Ӧ�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
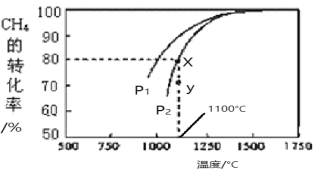
��ѹǿP2_____P1���� ��>������<������ԭ��________________________________________________________________________________��
��ѹǿΪP1ʱ����Y�㣺v(��)_______v(��)������>������<������=������
����X���Ӧ�¶��µĸ÷�Ӧ��ƽ�ⳣ��K=____________����������������λС����
��3��CO���Ժϳɶ����ѣ�CO(g)+4H2(g)![]() CH3OCH3(g)��H2O(g) ��H<0�������ѿ�����Ϊȼ�ϵ�ص�ԭ�ϡ����ô�ȼ�ϵ����ʯīΪ�缫���1L��0.5mol/L��CuSO4��Һ������ͨ��0.1mol����ʱ��������Һ������䣬��������ҺpH=______________������������ϲ���O2_______________L������С�������λ����
CH3OCH3(g)��H2O(g) ��H<0�������ѿ�����Ϊȼ�ϵ�ص�ԭ�ϡ����ô�ȼ�ϵ����ʯīΪ�缫���1L��0.5mol/L��CuSO4��Һ������ͨ��0.1mol����ʱ��������Һ������䣬��������ҺpH=______________������������ϲ���O2_______________L������С�������λ����
���𰸡�CH4(g)+2O2(g)=CO2(g)+2H2O(l) ![]() =-890.31kJ��mol-1>ͬһ�¶���ת����P1����P2�����÷�Ӧ�������������ķ�Ӧ����Сѹǿƽ��������Ӧ�����ƶ�������P2>P1<1.6410.56
=-890.31kJ��mol-1>ͬһ�¶���ת����P1����P2�����÷�Ӧ�������������ķ�Ӧ����Сѹǿƽ��������Ӧ�����ƶ�������P2>P1<1.6410.56
��������
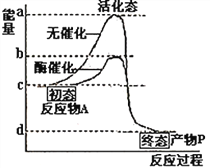
��1�����ݸ�˹���ɿɵ�CH4��ȼ����Ϊ��-607.31kJ/mol+��-566.0kJ/mol����2=-890.31kJ��mol-1������ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ![]() =-890.31kJ��mol-1����2����CO2(g)��CH4(g)
=-890.31kJ��mol-1����2����CO2(g)��CH4(g) ![]() 2CO(g)��2H2(g)��һ�����������Ŀ����ķ�Ӧ��ѹǿԽ��CH4��ת����ԽС������ͬһ�¶���CH4��ת������P1�����¸���ѹǿP1��С���ʴ�Ϊ��>��ͬһ�¶���ת����P1����P2�����÷�Ӧ�������������ķ�Ӧ����Сѹǿƽ��������Ӧ�����ƶ�������P2>P1������ͼ���֪����ѹǿΪP1ʱ��Y��÷�Ӧ������������Ӧ������ƽ��Ĺ����У���v(��) > v(��)������ͼ���֪��x��CH4��ת����Ϊ80%������ƽ��״̬��CO2(g)��CH4(g) ��CO(g)��H2(g)�ֱ�Ϊ0.02mol/L��0.02mol/L��0.16 mol/L��0.16 mol/L����ƽ�ⳣ��K=
2CO(g)��2H2(g)��һ�����������Ŀ����ķ�Ӧ��ѹǿԽ��CH4��ת����ԽС������ͬһ�¶���CH4��ת������P1�����¸���ѹǿP1��С���ʴ�Ϊ��>��ͬһ�¶���ת����P1����P2�����÷�Ӧ�������������ķ�Ӧ����Сѹǿƽ��������Ӧ�����ƶ�������P2>P1������ͼ���֪����ѹǿΪP1ʱ��Y��÷�Ӧ������������Ӧ������ƽ��Ĺ����У���v(��) > v(��)������ͼ���֪��x��CH4��ת����Ϊ80%������ƽ��״̬��CO2(g)��CH4(g) ��CO(g)��H2(g)�ֱ�Ϊ0.02mol/L��0.02mol/L��0.16 mol/L��0.16 mol/L����ƽ�ⳣ��K=![]() =1.64���ʴ�Ϊ1.64����3���ö��Ե缫���CuSO4��Һʱ�����ĵ缫��ӦʽΪ��2H2O-4e-=O2��+4H+���ɷ���ʽ�ļ�������ϵ�ɵã���ת�Ƶĵ���Ϊ0.1molʱ��������n��H+��=0.1mol����Ũ��Ϊ0.1mol/L��pH=1��n��O2��=0.1mol��4=0.025mol�����Ϊ0.025mol��22.4L/mol=0.56L���ʴ�Ϊ��1��0.56��
=1.64���ʴ�Ϊ1.64����3���ö��Ե缫���CuSO4��Һʱ�����ĵ缫��ӦʽΪ��2H2O-4e-=O2��+4H+���ɷ���ʽ�ļ�������ϵ�ɵã���ת�Ƶĵ���Ϊ0.1molʱ��������n��H+��=0.1mol����Ũ��Ϊ0.1mol/L��pH=1��n��O2��=0.1mol��4=0.025mol�����Ϊ0.025mol��22.4L/mol=0.56L���ʴ�Ϊ��1��0.56��

 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�����Ŀ��ʵ�������̷�(FeSO4��7H2O)�Ʊ���Ѫ���ʰ�������[(NH2CH2COO)2Fe] �й���������
�ʰ���(NH2CH2COOH) | ������ | �ʰ������� |
������ˮ�������Ҵ��� ���Ի����� | ������ˮ���Ҵ�����ǿ���� �ͻ�ԭ�� | ������ˮ�� �������Ҵ� |
ʵ����̣���.���ƺ�0.10mol FeSO4���̷���Һ��
��.�Ʊ�FeCO3�������ƺõ��̷���Һ��200mL 1.1mol��L-1NH4HCO3��Һ��ϣ���Ӧ��������˲�ϴ�ӳ�����
��.�Ʊ�(NH2CH2COO)2Fe��ʵ��װ������ͼ���гֺͼ���������ʡ�ԣ�����ʵ���õ��ij����ͺ�0.20 mol�ʰ����ˮ��Һ��Ϻ����C�У�Ȼ������A�еķ�Ӧ��C�п����ž������ŵ�����������Һ�����ȡ���Ӧ��������ˣ���Һ�������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ���ش��������⣺

��1��ʵ��I�У�ʵ���������̷���Һ����������ϡ������ʱ�����Լ�Ӧ�ù���_______
��2��ʵ��II�У��Ʊ�FeCO3ʱӦ��_______��Һ�л�������________��Һ�ӱ߽��裬���ߵ��Լ��μ�˳����ܲ����ĺ����__________________���ɳ��������ӷ���ʽΪ________________
��3��ȷ��C�п����ž���ʵ��������______________
��4��������������Һһ����ɵ�����Һ��pH�ٽ�FeCO3�ܽ⣬��һ��������___________
��5��ϴ��ʵ����еõ��ij�������ѡ�õ����ϴ���Լ���___________________��������ţ�
A����ˮ B���Ҵ���Һ C����������Һ
��6������Ʒ������Ϊ17.34g,�����Ϊ________%��