��Ŀ����
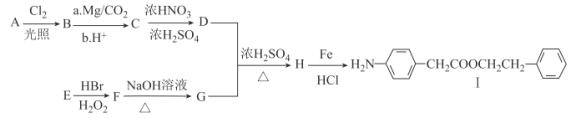
����Ŀ����֪����A ��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����2CH3CHO��O2 ![]() 2CH3COOH��F �Ǹ߷��ӻ�������� A Ϊ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
2CH3COOH��F �Ǹ߷��ӻ�������� A Ϊ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��

�ش��������⣺
��1��A �ĵ���ʽΪ______���ṹ��ʽΪ_______��
��2��B��D �����еĹ��������Ʒֱ���_______��_______��
��3����Ӧ�١��ݵķ�Ӧ����ȡ����Ӧ���ǣ�_____��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ����_____����______����_______��
��5�����������У�����ͨ�� A �ӳɷ�Ӧ�õ�����_____������ţ���
A��CH3CH3
B��CH3CHCl2
C��CH3CH2OH
D��CH3CH2Br
���𰸡�![]() CH2=CH2 �ǻ� �Ȼ� �� CH2=CH2+H2O
CH2=CH2 �ǻ� �Ȼ� �� CH2=CH2+H2O![]() CH3CH2OH CH3CH2OH+CH3COOH
CH3CH2OH CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O nCH2=CH2
CH3COOCH2CH3+H2O nCH2=CH2![]()
![]() ACD
ACD
��������
A��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ������ʯ�ͻ���ˮƽ����A����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����B����B���Ҵ����Ҵ�������������������Ӧ����C����C����ȩ��C������������������Ӧ����D��D�����ᣬ�Ҵ������ᷢ��������Ӧ��������������F�Ǹ߷��ӻ������A�����Ӿ۷�Ӧ����F��FΪ����ϩ���ݴ˷���������⡣
(1)��������������֪��AΪ��ϩ����ṹ��ʽΪCH2=CH2������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��CH2=CH2��
��CH2=CH2��
(2)BΪ�Ҵ����ṹ��ʽΪCH3CH2OH�����еĹ�����Ϊ�ǻ���DΪ���ᣬ�ṹ��ʽΪCH3COOH�����еĹ�����Ϊ�Ȼ����ʴ�Ϊ���ǻ����Ȼ���
(3)��������������Ӧ��Ϊ�ӳɷ�Ӧ����Ӧ�ڡ���Ϊ������Ӧ����Ӧ��Ϊ������Ӧ����ȡ����Ӧ����Ӧ��Ϊ�Ӿ۷�Ӧ���ʴ�Ϊ���ܣ�
(4)��Ӧ��Ϊ��ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����ѧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH����Ӧ��Ϊ�Ҵ������ᷢ��������Ӧ����������������Ӧ����ʽΪCH3CH2OH+CH3COOH
CH3CH2OH����Ӧ��Ϊ�Ҵ������ᷢ��������Ӧ����������������Ӧ����ʽΪCH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O����Ӧ��Ϊ��ϩ�����Ӿ۷�Ӧ�õ�����ϩ����Ӧ����ʽΪnCH2=CH2
CH3COOCH2CH3+H2O����Ӧ��Ϊ��ϩ�����Ӿ۷�Ӧ�õ�����ϩ����Ӧ����ʽΪnCH2=CH2![]()
![]() ���ʴ�Ϊ��CH2=CH2+H2O
���ʴ�Ϊ��CH2=CH2+H2O![]() CH3CH2OH��CH3CH2OH+CH3COOH
CH3CH2OH��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��nCH2=CH2
CH3COOCH2CH3+H2O��nCH2=CH2![]()
![]() ��
��
(5)A��CH2=CH2��H2�ӳɵõ�CH3CH3��Aѡ���������⣻
B��CH2=CH2����ֱ�Ӽӳɵõ�CH3CHCl2��Bѡ��������⣻
C��CH2=CH2��H2O�����ӳɵõ�CH3CH2OH��Cѡ���������⣻
D��CH2=CH2��HBr�����ӳɵõ�CH3CH2Br��Dѡ���������⣻
�ʴ�Ϊ��ACD��

����Ŀ��������Ԫ�ص����ӣ�![]() ������ͬ�ĵ��Ӳ�ṹ�����й�ϵ�в���ȷ���ǣ� ��
������ͬ�ĵ��Ӳ�ṹ�����й�ϵ�в���ȷ���ǣ� ��
A.ԭ�Ӱ뾶:X>W>Y>Z
B.��ԭ�ԣ�![]()
C.��������![]()
D.���Ӱ뾶��![]()
����Ŀ����.���ڿ����о��õ���Ƭ 5.4g Ͷ��ʢ�� 500mL0.5molL-1 ������Һ���ձ��и���Ƭ�����ᷴӦ���������������뷴Ӧʱ�������ͼ��ʾ��������������ʾ���ش��������⣺

��1�������� 0��a �β�����������ԭ��______�� �����ӷ���ʽ��ʾΪ______��
��2�������� b��c �β����������������ӽϿ����Ҫԭ��______��
��3������Һ�м����������ʣ��ܼӿ�������ѧ��Ӧ���ʵ���______��
A ����ˮ B �������� C �����Ȼ�����Һ D Ũ���� E ��������ͭ��Һ��
��.�� 2 L �ܱ������У�800 ��ʱ��Ӧ 2NO��O2![]() 2NO2 ��ϵ�У�n��NO����ʱ��ı仯���±���
2NO2 ��ϵ�У�n��NO����ʱ��ı仯���±���
ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��NO��/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��4����ͼ��ʾ������Ũ�ȵı仯���ߣ�B �㴦��v������_______v���棩�����á����ڡ���С�ڡ����ڡ���գ���

��5����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����______��
a ������ѹǿ���ֲ��� b v��NO����2v��O2��
c �����ڵ��ܶȱ��ֲ��� d 2v����NO2����v����O2��