��Ŀ����
��Ҫ��ش��������⣺
��1���Ȼ�������CrO2Cl2���۵㣺-96.5�棬�е㣺117�棬����CH3COCH3����ͪ����CS2�Ȼ��ܣ��ݴ��Ʋ�CrO2Cl2��������
��2����˵��H2SO4��H2SO3����ǿ��ԭ��
��3��H2S��H2O2����Ҫ�������ʱȽ����£�
H2S��H2O2����Է���������ͬ����������������ʲ������Ҫԭ����
��1���Ȼ�������CrO2Cl2���۵㣺-96.5�棬�е㣺117�棬����CH3COCH3����ͪ����CS2�Ȼ��ܣ��ݴ��Ʋ�CrO2Cl2��������
����
����
���壮��֪CS2��NO2+��Ϊ�ȵ����壬�� NO2+�����к��Цм���ĿΪ2NA
2NA
����2����˵��H2SO4��H2SO3����ǿ��ԭ��
����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ
����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ
����3��H2S��H2O2����Ҫ�������ʱȽ����£�
| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
H2O2����֮����γ�����������۷е�ߣ�H2O2��ˮ����֮����γ�����������ܽ�ȴ�
H2O2����֮����γ�����������۷е�ߣ�H2O2��ˮ����֮����γ�����������ܽ�ȴ�
����������1�����Ӿ�����۷е�ϵͣ�����̼��NO2+�Ľṹ���ƣ����ݶ���̼�Ľṹȷ��NO2+�Цм�������
��2������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
��3�����Ӱ�����ʵķе���ܽ��ԣ�������������ʷе�ϸߡ��ܽ��Խ�ǿ��
��2������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
��3�����Ӱ�����ʵķе���ܽ��ԣ�������������ʷе�ϸߡ��ܽ��Խ�ǿ��
����⣺��1�����Ӿ�����۷е�ϵͣ��Ȼ��������۷е�ϵͣ��������л��ܼ����������ڷ��Ӿ��壻
����̼��NO2+�Ľṹ���ƣ����ݶ���̼�Ľṹ֪NO2+����2���м�������1mol NO2+�к��Цм���ĿΪ2NA���ʴ�Ϊ�����ӣ�2NA��
��2������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
�ʴ�Ϊ������H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
��3��OԪ�طǽ����Խ�ǿ����Ӧ���⻯�����γ����������ˮ����֮��Ҳ�����γ��������Ԫ�غ�ˮ���Ӽ䲻���γ����������H2O2�ķе��H2S�ߣ�
�ʴ�Ϊ��H2O2����֮����γ�����������۷е�ߣ�H2O2��ˮ����֮����γ�����������ܽ�ȴ�
����̼��NO2+�Ľṹ���ƣ����ݶ���̼�Ľṹ֪NO2+����2���м�������1mol NO2+�к��Цм���ĿΪ2NA���ʴ�Ϊ�����ӣ�2NA��
��2������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
�ʴ�Ϊ������H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
��3��OԪ�طǽ����Խ�ǿ����Ӧ���⻯�����γ����������ˮ����֮��Ҳ�����γ��������Ԫ�غ�ˮ���Ӽ䲻���γ����������H2O2�ķе��H2S�ߣ�
�ʴ�Ϊ��H2O2����֮����γ�����������۷е�ߣ�H2O2��ˮ����֮����γ�����������ܽ�ȴ�
���������⿼���˷��Ӿ�����������ʣ�ͬ��Ԫ�غ��������Բ�ͬ��ԭ��������������ʵ�Ӱ�죬��Ŀ�漰��֪ʶ��϶࣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
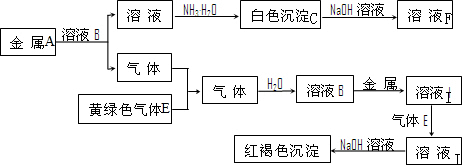
 A��B��C��D�����ɶ�����Ԫ����ɵij������ʣ�����A��B��C����ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ����
A��B��C��D�����ɶ�����Ԫ����ɵij������ʣ�����A��B��C����ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ���� H++CN-��H2O
H++CN-��H2O H++OH-��CN-+H2O
H++OH-��CN-+H2O HCN+OH-
HCN+OH-