��Ŀ����
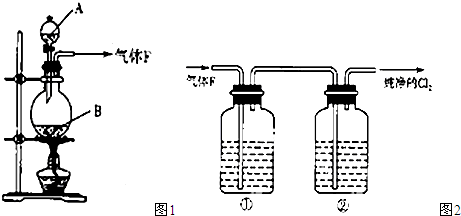
��ͼ1��ʾ��ij��ȤС��ͬѧ��ͭƬ����ϡ���ᣬ���ֿ�ʼʱ���ݲ������ʷdz�����һ��ʱ����������Լӿ죬��ƿ����Һ��dz��ɫ�����ϼ��Һ���Ϸ���������ɫҲ�ڲ��ϼ����С��ͬѧ��ͨ��ʵ��̽����Ӧ���ʱ仯��ԭ��

ͼ 1 ͼ 2
(1) ͼ1��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ ��
�����ӷ���ʽ��ʾNaOH��Һ��������� ��
(2) С��ͬѧ��������¼��貢���ʵ��̽����
��. ��ͬѧ��Ϊ�Ƿ�Ӧ���ȵ�����Һ�¶��������£���ɴ�ʵ�黹��Ҫ�������� ��
�ⶨ��Ӧ��������Һ��ͬʱ����¶ȣ�������±���
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 35 | 50 | 60 | 70 | 80 |
| �¶�/�� | 25 | 26 | 26 | 26 | 26 | 26 | 26.5 | 27 | 27 | 27 | 27 |
���ʵ��Ŀ�ĺͱ������ݣ���ó��Ľ����� ��
��. ��ͬѧ��Ϊ���ɵ�Cu2+�Է�Ӧ�д����ã�Ϊ��֤�˼��裬ȡA��B��֧�Թֱܷ���������ͭƬ��ϡ���ᣬ��ô�����������һ֧�Թ��м��������� ������ţ���
A. ����ͭ���� B. ����ͭ��Һ C. ����ͭ���� D. ����ͭ��Һ
Ȼ��Ա���֧�Թܵķ�Ӧ���������������ͬ����˵ó����ۣ�Cu2+�����Ƿ�Ӧ�Ĵ�����
��. ��ͬѧ���������ƲⷴӦ�����л������� ���ɣ�������Ϊ�����ʶԷ�Ӧ�д����ã���ͼ2��ʾ��ʵ���б�ͬѧ��a��ͨ������ʺ�������в��������������Կ����ҹܡ�С��ͬѧ�ó������ۣ��������ʶ�ͭ��ϡ����ķ�Ӧ�д����á�
��3��ʵ����������Թ�����Һ����ɫ����������ɫ������ͬѧ��Ϊ�Ǹ���Һ������ͭ�����������ϸ����£���һ����ͬѧ��Ϊ�Ǹ���Һ���ܽ���ͨ������ʡ��������һ��ʵ�鷽����֤�����ּ�����ȷ��(д��ʵ�������ʵ������ͽ���)
��
(1) 3Cu + 8H+ + 2NO3− === 3Cu2+ + 2NO�� + 4H2O ��2�֣�
NO + NO2 + 2OH− === 2NO2− + H2O��2�֣�
(2) ��. �¶ȼ� ��2�֣� �¶Ȳ����������Լӿ����Ҫԭ��2�֣�
��. A��2�֣� ��. NO2 ��2�֣�
(3) ���ȸ���ɫ��Һ���۲���ɫ�仯
���������ɫ��Һ��ͨ�뵪�����۲���ɫ�仯��������ͭ��Һ��ͨ��Ũ������ͭ��Ӧ���������壬�۲���ɫ�仯����2�֣�

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�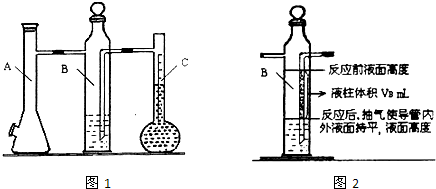
ʵ�鲽�裺
��1��װ��û�ѧ��Ӧ��������ⶨ�ǣ��������Լ�飮
��2����ɰƤ��ȥþ������������Ȼ���ȡ0.100g��0.110g��þ���������ݼ�¼�ڱ���
��3��ȡ��Aƿ���Ͽڵ���Ƥ������С�ձ�����20mLˮ���ٰ��ѳ�����þ���ӵ�Aƿ�ĵײ�������Ƥ���������Ͽڣ�
��4����ע������Aƿ���Ͽڴ�������ʹBƿ��������Һ���ƽ��
��5����ע������ȡ10mL 3mol/L���ᣬ����ͷ����Aƿ���Ͽ���Ƥ����������ע��Aƿ��ע���Ѹ�ٰγ���ͷ��
��6����þ����ȫ��Ӧ��ȡCƿ��Һ�������������ݼ�¼�ڱ���
��7����ע������Aƿ���Ͽڴ�������ʹBƿ�е�������Һ���ƽ����¼������������������ݼ�¼�ڱ���
�ظ������������еڶ���ʵ�飬����żȻ��
��������ʵ�鷽���ش��������⣺
ʵ�����¶ȣ�25�棬ѹǿ��101kPa����������1mol�������������ֵ��Ϊ24.5L
��1������װ�ü������Լ�飺��Aƿ���Ͽ�����������
��2��B����װҺ��һ����
��3��ʵ���������£��¶ȣ�25�棨þԪ�ص����ԭ������Ϊ24.3��
| ʵ����� | m��Mg��/g | �������/mL | Һ����ƿ��Һ�����/mL | ����������/mL | �������/mL | ����1mol�����/L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | X | |
| 2 | 0.115 | 10.0 | 121.0 | 8.0 |
�ڼ���1mol�����������ʵ���ƽ��ֵ=
�ۼ���ʵ������ʵ��ֵ-����ֵ��/����ֵ��100%=
���������صĿ���ԭ��
A��þ���к��и������Ӧ������
B��û�г�ȥþ�����������þ
C��þ���к���������
D������ϡ�������
��4������ͬѧ��ʵ���в�õ�����ƫ�ߣ���¼����ʱ�ѻָ������£���Ϊ�ˣ�ij����ȤС���ͬѧ�Դ�ʵ�鷽������������������飺
��A��Bƿ�������к���ˮ�������ӵ����������������ˮ�������ü��������������
��Bƿ�е��ܣ�ͼ2����Ӱ����VB����Һ�������ڷ�Ӧ��Ϊ������ռ�ݣ����ü��������������
����Ϊ���ǵ������������
| ʵ����� | m��Mg�� g |
�������mL | Һ����ƿ��Һ�����mL | ����������mL | Bƿ��һ��Һ�����mL | ˮ������ٷֺ��� | ����1mol�����L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | VB | a% |

 CH3COOH+OH-
CH3COOH+OH-