��Ŀ����
��12�֣�
̼����ĺ���Ӱ��������ܣ�̼��������һ�ֲⶨ�����ǽ�������̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
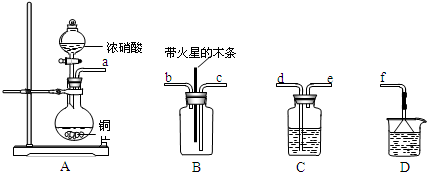
��1������װ��A���ڸ�����x�˸�����̼����ת��ΪCO2��SO2��

������a�ijɷ���____________________��
��������������FeS��ʽ���ڣ�A�з�Ӧ��3FeS+5 O2����1____+3_____��
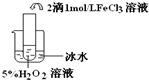
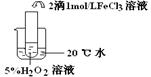
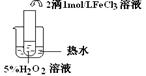
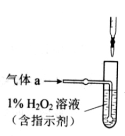
��2��������aͨ�������װ���У�����ͼ�������õζ����ⶨ��ĺ�����
��H2O2����SO2�Ļ�ѧ����ʽ��_________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mLNaOH��Һ��������1mLNaOH��Һ�൱���������Ϊy�ˣ���ø������������������_________________��
��3��������aͨ���̼װ���У�����ͼ���������������ⶨ̼�ĺ�����
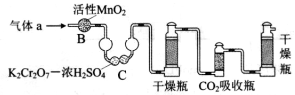
������aͨ��B��C��Ŀ����_____________________��
�ڼ��������̼������������Ӧ������������__________________��
̼����ĺ���Ӱ��������ܣ�̼��������һ�ֲⶨ�����ǽ�������̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
��1������װ��A���ڸ�����x�˸�����̼����ת��ΪCO2��SO2��

������a�ijɷ���____________________��
��������������FeS��ʽ���ڣ�A�з�Ӧ��3FeS+5 O2����1____+3_____��
��2��������aͨ�������װ���У�����ͼ�������õζ����ⶨ��ĺ�����
��H2O2����SO2�Ļ�ѧ����ʽ��_________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mLNaOH��Һ��������1mLNaOH��Һ�൱���������Ϊy�ˣ���ø������������������_________________��
��3��������aͨ���̼װ���У�����ͼ���������������ⶨ̼�ĺ�����


������aͨ��B��C��Ŀ����_____________________��
�ڼ��������̼������������Ӧ������������__________________��
��1����CO2��SO2��O2����Fe3O4��SO2
��2����H2O2+SO2=H2SO4���� zy/x
��3���ٳ�ȥSO2��CO2�ⶨ�ĸ���
������CO2����ǰ������ƿ������
��2����H2O2+SO2=H2SO4���� zy/x
��3���ٳ�ȥSO2��CO2�ⶨ�ĸ���
������CO2����ǰ������ƿ������
�����������1���ٸ����е�̼������װ��A�б�����ΪCO2��SO2����a�ijɷ�ΪCO2��SO2�Լ�δ��Ӧ��O2��
��FeS�еģ�-2�۵�������ΪSO2��+2�۵�Fe������Ϊ+3�۵��������������ѧ����������֪����ӦΪFe3O4��SO2���ʷ���ʽΪ3FeS+5O2=Fe3O4+3SO2��
��2����H2O2���������ԣ�������SO2ʹS�Ļ��ϼ�����Ϊ+6�ۣ�����Һ�з�Ӧ����ӦΪ���ᣬ�ʷ�Ӧ����ʽΪ��H2O2+SO2=H2SO4����1mL NaOH�൱��yg S����z mL NaOH�൱�� zy g S�������Ʒ�������������Ϊzy/x ��
��3���ٲⶨ̼�ĺ����轫����a�е�SO2��ȥ����װ��B��C�������dz�ȥSO2���ڼ���̼����������������CO2���������з������������CO2����ƿ������CO2����ǰ���������

��ϰ��ϵ�д�
�����Ŀ