��Ŀ����
��1��ͬ��ͬѹ�£���������A��B�����֮��Ϊ2��1������֮��Ϊ8��5����A��B���ܶ�֮��Ϊ______��Ħ������֮��Ϊ______��
��2���ڱ�״����a.6.72L CH4���塡 b.3.01��1023��HCl������ӡ���c.13.6g�� H2S���塡d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ���______��
�ڱ�״����������������______��
���������������______��
�⣺��1��ͬ��ͬѹ�£���������A��B�����֮��Ϊ2��1������֮��Ϊ8��5��
�����ʵ����ֱ�Ϊ2mol��1mol�������ֱ�Ϊ8g��5g����
Ħ������֮��Ϊ ��
��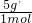 =4��5��
=4��5��
����pM=��RT��֪���ܶ���Ħ�����������ȣ����ܶ�֮��Ϊ4��5��
�ʴ�Ϊ��4��5��4��5��
��2�����ڱ�״����a.6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3��
CH4��������ʵ���Ϊ =0.3mol��HCl��������ʵ���Ϊ
=0.3mol��HCl��������ʵ���Ϊ =0.5mol��
=0.5mol��
H2S��������ʵ���Ϊ =0.4mol��NH3�����ʵ���Ϊ0.2mol��
=0.4mol��NH3�����ʵ���Ϊ0.2mol��
��0.5��0.4��0.3��0.2����������������ʵ���Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
����������ʵ��������ȣ���������������Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
��CH4���������Ϊ0.3mol��16g/mol=4.8g��HCl������Ϊ0.5mol��36.5g/mol=18.25g��
H2S������Ϊ13.6g��NH3������Ϊ0.2mol��17g/mol=3.4g��
���������������Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
��������1��ͬ��ͬѹ�£����֮�ȵ������ʵ���֮�ȣ�n= ��pM=��RT�����㣻
��pM=��RT�����㣻
��2������n=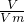 =
=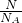 =
= ���������ʵ�������������������ʵ��������������
���������ʵ�������������������ʵ��������������
���������⿼�����ʵ������йؼ��㣬��ȷn=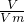 =
=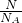 =
= ��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ��
��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ��
�����ʵ����ֱ�Ϊ2mol��1mol�������ֱ�Ϊ8g��5g����
Ħ������֮��Ϊ
 ��
��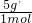 =4��5��
=4��5������pM=��RT��֪���ܶ���Ħ�����������ȣ����ܶ�֮��Ϊ4��5��
�ʴ�Ϊ��4��5��4��5��
��2�����ڱ�״����a.6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3��
CH4��������ʵ���Ϊ
 =0.3mol��HCl��������ʵ���Ϊ
=0.3mol��HCl��������ʵ���Ϊ =0.5mol��
=0.5mol��H2S��������ʵ���Ϊ
 =0.4mol��NH3�����ʵ���Ϊ0.2mol��
=0.4mol��NH3�����ʵ���Ϊ0.2mol����0.5��0.4��0.3��0.2����������������ʵ���Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
����������ʵ��������ȣ���������������Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
��CH4���������Ϊ0.3mol��16g/mol=4.8g��HCl������Ϊ0.5mol��36.5g/mol=18.25g��
H2S������Ϊ13.6g��NH3������Ϊ0.2mol��17g/mol=3.4g��
���������������Ϊb��c��a��d���ʴ�Ϊ��b��c��a��d��
��������1��ͬ��ͬѹ�£����֮�ȵ������ʵ���֮�ȣ�n=
 ��pM=��RT�����㣻
��pM=��RT�����㣻��2������n=
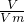 =
=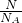 =
= ���������ʵ�������������������ʵ��������������
���������ʵ�������������������ʵ�����������������������⿼�����ʵ������йؼ��㣬��ȷn=
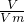 =
=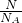 =
= ��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ��
��pM=��RT��ͬ��ͬѹ����������ʵ����Ĺ�ϵ�ȼ��ɽ�� 
��ϰ��ϵ�д�
�����Ŀ