��Ŀ����
(14��)������������ƫͷʹ����Ч��ҩ��ij�о�С�鿪�������ºϳ�·�����ϳɸ�ҩ��
��֪:��A����Է�������Ϊ104,1 mol A��������̼�����Ʒ�Ӧ����44.8 L����(��״��);
��B�Ľṹ�к���ȩ��; ��C��һ�������������л���D;
��RCHO+HOOC��CH2COOH RCH=C(COOH)2+H2O
RCH=C(COOH)2+H2O
RCH=C(COOH)2 RCH=CHCOOH+CO2������ش��������⡣
RCH=CHCOOH+CO2������ش��������⡣
��1��A�ķ���ʽ������������,B�Ľṹ��ʽΪ��������������
��2��C���ܷ����ķ�Ӧ��_________(�����)��
| A��������Ӧ | B��ˮ�ⷴӦ | C����ȥ��Ӧ | D��������Ӧ |
��4�� E������������������ ��
��5�� ��������������D��ͬ���칹�干������������,�����ں˴Ź���������ֻ�������������ʵĽṹ��ʽΪ����������������������
�ٱ�����ֻ������ȡ����; �ڱ����ϵ�һ�ȴ���ֻ������;
��1 mol��ͬ���칹����������̼�����Ʒ�Ӧ����2 mol CO2��
��1�� C3H4O4;  ; ��2�� A��
; ��2�� A��
��3��
��4�� ̼������;
��5��4;  ��
�� ��
�� ��
��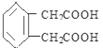 );
);
���������������1������A����Է������������̼�����Ʒ�Ӧ�����ʵ����Ĺ�ϵ����B��Ӧ�õ���C�Ľṹ��ʽ����֪A�ķ���ʽ��C3H4O4��B�Ľṹ��ʽΪ ����2������C
����2������C �ķ��ӽṹ�к���C=C˫�������ǻ��������ܹ�����������Ӧ�������Ȼ����ǻ��������ܹ�����������Ӧ�������Ƿ��ǻ�����˲��ܷ�����ȥ��Ӧ�������������ܷ���ˮ�ⷴӦ����ѡ��ΪA��D����3������D��C�ڷ�����������һ��CO2����˸÷�Ӧ�Ƿ��ӵ����Ȼ���Ӧ������ʽΪ��
�ķ��ӽṹ�к���C=C˫�������ǻ��������ܹ�����������Ӧ�������Ȼ����ǻ��������ܹ�����������Ӧ�������Ƿ��ǻ�����˲��ܷ�����ȥ��Ӧ�������������ܷ���ˮ�ⷴӦ����ѡ��ΪA��D����3������D��C�ڷ�����������һ��CO2����˸÷�Ӧ�Ƿ��ӵ����Ȼ���Ӧ������ʽΪ�� �������ڵ�������ֻ����һ��Na+.������ʵ�����ǿ��˳����D��Ӧ������EӦ����̼�����ơ�D��̼��������Һ�ų���Ӧ�õ�������:
�������ڵ�������ֻ����һ��Na+.������ʵ�����ǿ��˳����D��Ӧ������EӦ����̼�����ơ�D��̼��������Һ�ų���Ӧ�õ�������: , ��5�� ����������D��ͬ���칹�干�����֣����Ƿֱ���
, ��5�� ����������D��ͬ���칹�干�����֣����Ƿֱ��� ��
�� ��
�� ��
�� ��
��
���㣺�����л���Ľṹ�����ʡ��ת������ѧ����ʽ��ͬ���칹�����д��֪ʶ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
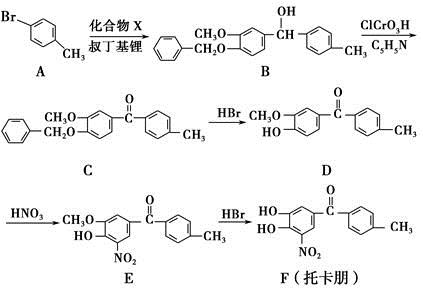
Сѧ��10����Ӧ����ϵ�д�(8��)�л���M�Ľṹ��ʽ��ͼ��ʾ��
(1)�л���M�ı����ϵ�һ�ȴ�����________�֡�
(2)1 mol M��������ˮ��ϣ�����Br2�����ʵ���Ϊ________mol��
(3)1 mol M������H2�ӳɣ�����H2________ mol��
(4)�����й�M��˵���в���ȷ����________��
| A���ڴ����������£�M����Һ�巢��ȡ����Ӧ |
| B��Mʹ��ˮ��ɫ��ԭ������ϩʹ��ˮ��ɫ��ԭ����ͬ |
| C��M��ʹ����KMnO4��Һ��ɫ |
| D��M�ķ���ʽΪC15H16 |
 ____________________ ��
____________________ �� ____________________
____________________ ��ע����������ʡ�ԡ�
��ע����������ʡ�ԡ�

 )���������ȡ��������ͬһ�������ϣ���������������ˮ�����ɵ����ֲ��ﶼֻ��4�ֲ�ͬ��ѧ�������⡣
)���������ȡ��������ͬһ�������ϣ���������������ˮ�����ɵ����ֲ��ﶼֻ��4�ֲ�ͬ��ѧ�������⡣ �Ǻϳ����˷ܼ���������м��壬��д����CH3CH2CH2Br��
�Ǻϳ����˷ܼ���������м��壬��д����CH3CH2CH2Br�� Ϊԭ��(ԭ����ͼ�����е��Լ������Լ���ѡ)�Ʊ��û�����ĺϳ�·������ͼ��
Ϊԭ��(ԭ����ͼ�����е��Լ������Լ���ѡ)�Ʊ��û�����ĺϳ�·������ͼ��