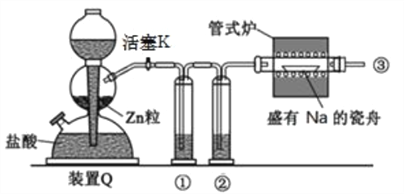
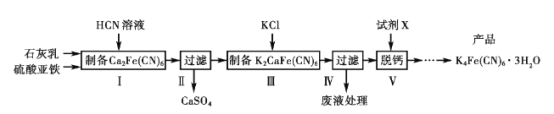
��Ŀ����
����Ŀ����������Ԫ�������������о�����Ҫ�����á���ش���������:
(1)��̬Scԭ�ӵļ۵����Ų�ͼΪ___________________��
(2)Zn2+��CN-��K+����ɻ�����K2Zn(CN)4,���д��ڵĻ�ѧ��������____________��Zn(CN)42-�е�����Ϊ_______����λԭ��Ϊ_____ ,���������������ȵ�����ķ���Ϊ___��
(3)��Brͬ����Ķ�����Ԫ����F��Cl,������Ԫ�صļ��⻯��HF��HCl��HBr�ķе�Ӹߵ��͵�˳��Ϊ___________������Ϊ______��
(4)Fe-Cr-Al�Ͻ����Ϊ����β������������,����β�������ɽ�NO2��ԭΪ����������,��ֹHNO3��������γ�,NO2�Ŀռ乹��Ϊ_____��HNO3������ǿ��HNO2��ԭ��Ϊ____________________��
(5)����ͭ�������������ѻ���ʽ,�侧���ṹ��ͼ��ʾ����֪�þ������ܶ�Ϊ��g. cm-3,��������Ϊanm,����٤������ΪNA,��ͭԭ�ӵ����ԭ�������ı���ʽΪ_______(�ú�������a��NA�ı���ʽ��ʾ)��

���𰸡�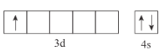 ���Ӽ������ۼ�����λ�� CN�� C N2��CO HF>HBr>HCl HF��HCl��HBr��Ϊ���Ӿ��壬HF����֮�������������HF�ķе���ߣ�HCl��HBr����Է���������HBr�ķе����HCl�� V�� HNO3��N�Ļ��ϼ�Ϊ��5�ۣ���N��������ǿ��HNO2��N��ʹ�ǻ���O��H���Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ��������H����������HNO3ǿ��HNO2
���Ӽ������ۼ�����λ�� CN�� C N2��CO HF>HBr>HCl HF��HCl��HBr��Ϊ���Ӿ��壬HF����֮�������������HF�ķе���ߣ�HCl��HBr����Է���������HBr�ķе����HCl�� V�� HNO3��N�Ļ��ϼ�Ϊ��5�ۣ���N��������ǿ��HNO2��N��ʹ�ǻ���O��H���Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ��������H����������HNO3ǿ��HNO2 ![]()
��������
(1)Scԭ��������21��Ԫ�أ����ڹ���Ԫ�أ��۵����Ų�ʽΪ3d14s2��Ȼ����д���۵����Ų�ͼ��
(2)�û��������������ڽ����������Ӽ���ʽ��ϣ�Ȼ�����������ɽ��з�����
(3)���Ӿ����۷е�ߵ͵��жϣ����Ƿ��з��Ӽ��������Է���������С�ǶȽ��з�����
(4)����VSEPRģ�ͽ��з��������ú���������ǿ�����ɽ��з�����
(5)�����ܶȵĶ�����з������㣻
(1) Scԭ��������21��Ԫ�أ����ڹ���Ԫ�أ��۵����Ų�ʽΪ3d14s2��Sc�۵����Ų�ʽͼΪ ��
��
(2)K2Zn(CN)4��K����Zn(CN)42��֮��������Ӽ���Zn2����CN��֮�乹����λ����CN����C��N֮����ڹ��ۼ�����˸�������к��л�ѧ��������Ϊ���Ӽ�����λ�������ۼ���Zn2���ṩ�չ����CN����C�ṩ�µ��Ӷԣ��������ΪCN������λԭ��ΪC�����ݵȵ�����Ķ��壬��CN���ȵ�����ķ���ΪN2��CO�ȣ�
(3)HF��HCl��HBr�����ڷ��Ӿ��壬HF���з��Ӽ������HCl��HBr�������Ӽ������HBr����Է�����������HCl��HBr�ķ��Ӽ�����������HCl����˷е��С˳����HF>HBr>HCl��
(4)NO2�к���2���Ҽ����µ��Ӷ���=![]() =0.5��������Ϊ����ԭ��N�ļ۲���Ӷ���Ϊ3��NO2�Ŀռ乹��ΪV�ͣ�HNO3��N�Ļ��ϼ�Ϊ��5�ۣ���N��������ǿ��HNO2��N��ʹ�ǻ���O��H���Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ��������H����������HNO3ǿ��HNO2��
=0.5��������Ϊ����ԭ��N�ļ۲���Ӷ���Ϊ3��NO2�Ŀռ乹��ΪV�ͣ�HNO3��N�Ļ��ϼ�Ϊ��5�ۣ���N��������ǿ��HNO2��N��ʹ�ǻ���O��H���Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ��������H����������HNO3ǿ��HNO2��
(5)���ݾ����Ľṹ��Cuԭ��λ�ڶ�������ģ�����Ϊ![]() =4������������Ϊ
=4������������Ϊ![]() g��mol��1�����������Ϊ(a��10��7)3cm3�������ܶȵĶ��壬
g��mol��1�����������Ϊ(a��10��7)3cm3�������ܶȵĶ��壬 ���Ƴ�M=
���Ƴ�M=![]() ��
��