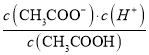��Ŀ����
����Ŀ��ʵ��������500mL 0.2mol/L��NaOH��Һ��
(1)����ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_____________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��____________��

(2)��д���������еĿհף�
���岽�����£�
�ټ�����Ҫ����NaOH���������___________g;
����������ƽ����NaOH���壻
�۽��ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬��____________�����£�
�ܽ�NaOH��Һ�ز�����ע��____________�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����_______cmʱ������____________�μ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
(3)ʹ������ƿǰ������е�һ��������_____________________��
(4)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L,ԭ�������___________��
A.ʹ����ֽ����NaOH���壻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ�
C.����ƿ��ԭ������������ˮ��
D.����ʱ���õ��������⣻
E.δ��ȴֱ��ת��������ƿ��������ã�
���𰸡�C �ձ� ������ 4.0g ��ȴ 500 mL����ƿ 1��2 ��ͷ�ι� ��© AB
��������
��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ����Ҫ��������Ȼ�������������ȷ����ȱ�ٵ��������ƣ�
��2���ٸ��ݻ�����ʽn = CV�����������m=n M��������������ƽ������ȷ��0.1 g����
������ƿ�������ȣ�
������ƿֻ��һ���̶��ߣ�ֻ���������������Ӧ���������Һ��
���ݶ��ݵIJ�����������
��3����������ƿ��ʹ��ע������������
��4���������������ļ�С����Һ��������ᵼ����ҺŨ�ȵ�ƫС��
��1��CΪ��Һ©������������Һ�����в���ʹ�õ���ƿ�ͷ�Һ©��������500mL 0.2 mol/LNaOH��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2��3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ������Ϊ���ձ�����������
�ʴ�Ϊ��C���ձ�����������
��2�������������m=CVM=0.2mol/L��0.5 L��40 g/mol=4.0 g��
�ʴ�Ϊ��4.0 g��
������ƿ����������NaOH����ˮ���ȣ�����ת��֮ǰ��Ҫ���Ѿ��ܽ�õ�NaOH��ȴ�����£�
�ʴ�Ϊ����ȴ
����һ������Ϊת�ƹ��̣�����ƿֻ��һ���̶��ߣ�ֻ���������������Ӧ���������Һ��
�ʴ�Ϊ��500 mL����ƿ��
������ˮע������ƿ��Һ����̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ���ڿ̶�������,
�ʴ�Ϊ��1��2����ͷ�ιܣ�
��3������ƿ��ƿ�������ƹ�������Ҫҡ�ȣ�Ϊ�˱���©Һ��ʹ������ƿǰ�����Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4�� ʵ����������������ҺŨ��Ϊ0.095mol/L��С��0.1mol/L����ʵ������������������������ļ�С����Һ���������
A. �����������׳��⣬����ֽ�����������ƹ��壬�ᵼ����������������С������ʹ�������ʵ���Ũ��ƫС����A�����������
B. �ܽ����ձ�δ�����ϴ�ӣ���ʹ����������ʧ�����ջᵼ����Һ���������Ƶ����ʵ���Ũ��ƫС����B�����������
C. ��ת�Ʋ����Ժ���Ҫ���ݼ�����ˮ���̶��ߣ��������ƿ��ԭ��������������ˮ����ʵ������Ӱ�죬��C�����������
D. ����ʱ���õ��������⣬��������ȡ�ù��������������ʹ��ʵ����ƫ��D�����������
E.����������δ��ȴֱ��ת��������ƿ���������ʱ��������Һ��ʵ�����ƫС������C = ![]() ���Կ���������ʹ��ʵ����ƫ��E�����������
���Կ���������ʹ��ʵ����ƫ��E�����������
��ѡAB��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�����Ŀ�������Ƶ���Ҫ�ɷ�����������������һ�ֳ����IJ���ҩ�ijͬѧΪ�����������Fe2+�Ĵ���,��Ʋ�����ʵ������:
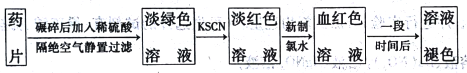
�ش���������:
��1��ʵ��������ҩƬ��Ҫ��������____________��
��2����KSCN��Һ��,��Һ�ʵ���ɫ,��ԭ�������___________������������ˮ��,������Ӧ�����ӷ���ʽΪ_________________��
��3������һ��ʱ���,��Һ����ɫ������ȥ�������Һ��ɫ��ԭ������2�ֲ���:
��� | ���� |
�� | ______ |
�� | _______ |
��4��ҽѧ�Ϸ���ά����C,�ɷ�ֹ�����������ӱ��������ɴ��Ʋ�ά����C����______�ԡ�
��5��������ÿ��Ӧ����14mg���ҵ���,���о���������ʳ����ȫ��ͨ�����ú�FeS04��7H2O��Ƭ����������,��������ÿ������ú�_____mgFeSO4��7H2O��Ƭ����