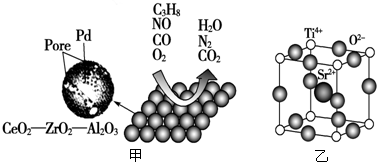
��Ŀ����
18�������Ѷ����������ж��ԣ����뼡�����������һ������С����������֮�ƣ������й�${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��˵������ȷ���ǣ�������| A�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Tiԭ���о�����22������ | |
| B�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti������������ | |
| C�� | �ֱ���${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��ɵĽ����ѵ��ʻ���Ϊͬλ�� | |
| D�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$TiΪͬһ���� |
���� A��ԭ�ӷ���ZAX�����½�Z���������������Ͻ�A������������X����Ԫ�ط��ţ�������=������-���������ݴ��жϣ�
B��ԭ�ӵ�������=�����������
C����������ͬ����������ͬ��ͬ��Ԫ�صIJ�ͬ��ԭ�ӻ�Ϊͬλ�أ�
D��${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��������ͬ��������ͬ����TiԪ�ز�ͬ��ԭ�ӣ�
��� �⣺A��${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti����������ͬ����22���������ֱ�Ϊ48-22=26��50-22=28����A����
B��${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti������ͬ��Ԫ�أ�������=������=22����B��ȷ��
C��${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��Ϊͬλ�أ���ɵĽ����ѵ�������ͬ�����ʣ���C����
D��${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��������ͬ��������ͬ����Ϊͬλ�أ���TiԪ�ز�ͬ�ĺ��أ���D����
��ѡB��
���� ���⿼��ԭ�ӷ��š��ṹ��λ�ù�ϵ��ͬλ�ؼ������ʣ��ѶȲ���ע�����֪ʶ�����գ�

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ
8��ij��ѧС��ͨ���������ϣ��������ͼ��ʾ�ķ����Ժ�������Ϊԭ�����Ʊ�NiSO4•7H20����֪ij�������ĺ����ϴ�����Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%����
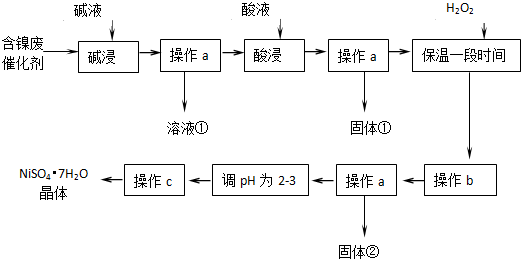
����������������������ʽ����ʱ��pH���£�
��1���������ʱ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2����Al2O3+2OH-�T2AlO2-+H2O��
��2���������ʱ�����������H2SO4���ѧʽ����
��3������H2O2ʱ������Ӧ�����ӷ���ʽΪH2O2+2Fe2++2H+=2Fe3++2H2O��
��4������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��3.2-7.1��
��5��NiS04•7H20�������Ʊ������أ�NiMH����������Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������ͣ�NiMH�е�M��ʾ���������Ͻ𣮸õ���ڳ��������ܷ�Ӧ�Ļ�ѧ����ʽ��Ni��OH��2+M�TNiOOH+MH����NiMH��طŵ�����У������ĵ缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH-��

����������������������ʽ����ʱ��pH���£�
| ������ | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| Fe��OH��2 | 7.6 | 9.7 |
| Ni��OH��2 | 7.1 | 9.2 |
��2���������ʱ�����������H2SO4���ѧʽ����
��3������H2O2ʱ������Ӧ�����ӷ���ʽΪH2O2+2Fe2++2H+=2Fe3++2H2O��
��4������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��3.2-7.1��
��5��NiS04•7H20�������Ʊ������أ�NiMH����������Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������ͣ�NiMH�е�M��ʾ���������Ͻ𣮸õ���ڳ��������ܷ�Ӧ�Ļ�ѧ����ʽ��Ni��OH��2+M�TNiOOH+MH����NiMH��طŵ�����У������ĵ缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH-��
6����1 mol��������˵�һ����������ˮ�����յõ�1L��Һ�����и����У������Ѵﵽ����ƽ��״̬���ǣ�������
| A�� | �����Ũ�ȴﵽ1 mol•L-1 | |
| B�� | H+��Ũ�ȴﵽ0.5 mol•L-1 | |
| C�� | ������ӵ�Ũ�ȡ���������ӵ�Ũ�ȡ�H+��Ũ�Ⱦ�Ϊ0.5 mol•L-1 | |
| D�� | ������ӵ�������ӵ����ʺ��������½�ϳɴ�����ӵ�������ȣ� |
13��������ɱ���ܱ������У���ӦmA��g��+nB��s��?qC��g���ﵽƽ���ѹ���������������A��ת���ʽ��ͣ�����˵���У���ȷ���ǣ�������
| A�� | ��m+n���ض�С��q | B�� | ��m+n���ض�����q | C�� | m�ض�С��q | D�� | m�ض�����q |
3��ʪ��ѧ����NPP-�����Ʊ�������������п������������п��Ʒ������FeO��Fe2O3��CuO���ʣ�Ϊԭ�ϣ����������п��������ξ�����ȥԭ���е����ʣ�Ȼ�������ü�ʽ̼��п������ջ�û�������п���仯ѧ����������ͼ��
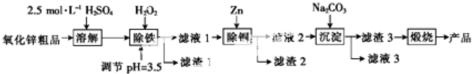
��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�������������ʽ����ʱ��pH�����
��1��ʵ������98%ŨH2SO4����=1.84g/cm3��������100ml 2.5mol/LϡH2SO4����IJ����������ձ�����ͷ�ιܡ�00mL����ƿ����Ͳ����������
��2����������ͼ��pH=12��Na2CO3��Һ�������ӵ�Ũ���ɴ�С��˳��Ϊc��CO32-����c��OH-����c��HCO3-����
��3������1��Fe��OH��3���ѧʽ����ͬ��������2��Cu��Zn������H2O2ʱ������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
��4�����������õ�ZnCO3•2Zn��OH��2•H2O�������ա���450��500���½��У������ա���Ӧ�Ļ�ѧ����ʽΪZnCO3•2Zn��OH��2•H2O$\frac{\underline{\;450��-500��\;}}{\;}$3ZnO+CO2��+3H2O����

��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�������������ʽ����ʱ��pH�����
| ���� | ��ʼ�� ����pH | ��ȫ�� ����pH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.7 | 3.2 |
| Cu2+ | 5.2 | 6.7 |
| Zn2+ | 6.8 | 9.0 |
��2����������ͼ��pH=12��Na2CO3��Һ�������ӵ�Ũ���ɴ�С��˳��Ϊc��CO32-����c��OH-����c��HCO3-����
��3������1��Fe��OH��3���ѧʽ����ͬ��������2��Cu��Zn������H2O2ʱ������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
��4�����������õ�ZnCO3•2Zn��OH��2•H2O�������ա���450��500���½��У������ա���Ӧ�Ļ�ѧ����ʽΪZnCO3•2Zn��OH��2•H2O$\frac{\underline{\;450��-500��\;}}{\;}$3ZnO+CO2��+3H2O����
7�����и���Ԫ���У��������ҵ�˳��ԭ������������Ԫ�ص���������ϼ�Ҳ�������ǣ�������
| A�� | Na��Mg��Al��Si | B�� | Na��Be��B��C | C�� | P��S��Cl��Ar | D�� | C��N��O��F |
8������˵������ȷ���ǣ�������
| A�� | ������Һ��һ����c��H+��=c��OH-��=1��10-7mol/L | |
| B�� | ��0.1mol/L��NaOH��Һ�м�����������ˮ����c��H+����c��OH-�������� | |
| C�� | 25��ʱ��pH=6����Һ�в����ܴ���NH3•H2O���� | |
| D�� | �����£�pH=8����Һ�п��ܴ���CH3COOH���� |