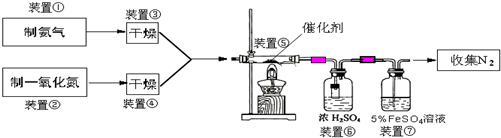
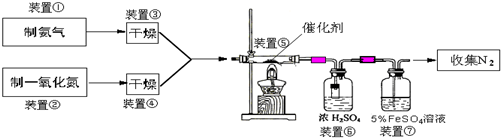
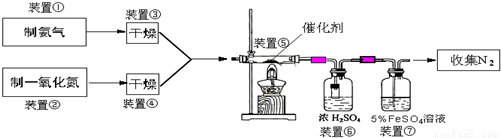
ΧβΡΩΡΎ»ί
NH3‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύ ±ΡήΜΙ‘≠NOxΈΣN2ΚΆH2OΘ§’β «ΡΩ«ΑΙζΆβœθΥα≥ßΫχ––Έ≤Τχ÷ΈάμΥυΤ’±ι≤…”ΟΒΡ“Μ÷÷ΖΫΖ®Θ°œ¬ΆΦ «Ρ≥–ΘΜ·―ß–Υ»Λ–ΓΉι…ηΦΤΒΡΡΘΡβΑ±ΤχΜΙ‘≠NOΒΡΉΑ÷ΟΘ° 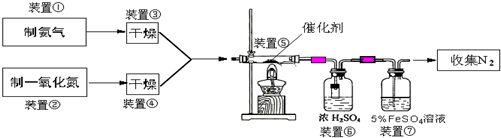
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©»τ÷Τ»ΓΑ±Τχ”ΟAΉΑ÷ΟΘ§‘ρΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______Θ°»τ”ΟBΉΑ÷Ο÷Τ»ΓΑ±ΤχΘ§‘ρΖ÷“Κ¬©ΕΖΚΆΉΕ–ΈΤΩ÷– ΔΖ≈ΒΡ“©ΤΖΖ÷±π «______Θ°

Θ®2Θ©»τ÷Τ»ΓNO”ΟΆΦCΉΑ÷ΟΘ§‘ρ÷Τ»ΓNOΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______Θ§”ΟΩ…≥ιΕ·ΒΡΆ≠ΥΩΤδ”≈Βψ «______Θ°
Θ®3Θ©ΉΑ÷ΟΔΏΒΡΉς”ΟΩ…Ρή «______Θ°
Θ®4Θ©Μν–‘―«ΗθΥαΆ≠Θ®Ω…–¥≥…xCuO?yCr2O3ΒΡ–Έ ΫΘ§xΓΔyΈΣ’ΐ’ϊ ΐΘ© «Α±ΤχΜΙ‘≠NO¥ΏΜ·ΦΝΘ°“―÷ΣCuNH4Θ®OHΘ©CrO4‘Ύ295ΓφΖ÷Ϋβ…ζ≥…Μν–‘―«ΗθΥαΆ≠¥ΏΜ·ΦΝΓΔ“Μ÷÷ΈόΕΨΤχΧεΦΑΥ°Θ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ______Θ°
Θ®5Θ©»τΫχ»κΉΑ÷ΟΔίΒΡNOΙ≤2688mLΘ®“―’έΥψΈΣ±ξΉΦΉ¥ΩωΘ§œ¬Ά§Θ©Θ§Α±ΤχΙΐΝΩΘ§ΉνΚσ ’Φ·ΒΫ±ξΉΦΉ¥Ωωœ¬2016mL N2Θ§‘ρNOΒΡΉΣΜ·¬ ΈΣ______Θ°
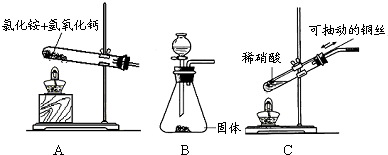
ΫβΘΚΘ®1Θ©‘ΎΦ”»»ΧθΦΰœ¬Θ§ Β―ι “≥Θ”Ο¬»Μ·οßΚΆ«β―θΜ·ΗΤΖ¥”Π÷Τ±ΗΑ±ΤχΘ§Ά§ ±…ζ≤欻̷ΗΤΚΆΥ°Θ§ΖΫ≥Χ ΫΈΣ2
NH4Cl+CaΘ®OHΘ©2 CaCl2+2NH3Γϋ+2H2OΘ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘ§
CaCl2+2NH3Γϋ+2H2OΘ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘ§
Ι ¥πΑΗΈΣΘΚ2NH4Cl+CaΘ®OHΘ©2 CaCl2+2NH3Γϋ+2H2OΘΜ≈®Α±Υ°ΓΔΦν ·Μ“ΘΜ
CaCl2+2NH3Γϋ+2H2OΘΜ≈®Α±Υ°ΓΔΦν ·Μ“ΘΜ
Θ®2Θ©Ά≠ΚΆœΓœθΥαΖ¥”Π…ζ≤ζœθΥαΆ≠ΚΆ“Μ―θΜ·ΒΣΘ§άκΉ”ΖΫ≥Χ ΫΈΣ3Cu+8H++2NO3-=3Cu2++2NOΓϋ+4H2OΘ§”ΟΩ…≥ιΕ·ΒΡΆ≠ΥΩ“Ή”ΎΩΊ÷ΤΖ¥”ΠΘ§≤ΌΉςΖΫ±ψ≤ΔΡήΫΎ‘Φ“©ΤΖΘ§
Ι ¥πΑΗΈΣΘΚ3Cu+8H++2NO3-=3Cu2++2NOΓϋ+4H2OΘΜΖ¥”ΠΩ…“‘Υφ ±ΙΊΆΘΓΔ≤ΌΉςΖΫ±ψΓΔΖ¥Η¥ Ι”ΟΓΔΫΎ‘Φ“©ΤΖΘΜ
Θ®3Θ©“Μ―θΜ·ΒΣ”κΕΰΦέΧζ–Έ≥…≈δΈΜΦϋΘ§»ή“Κ÷–Β≠¬Χ…ΪΒΡΕΰΦέΧζ±δ≥…ΉΊ…ΪΒΡ“Μ―θΜ·ΒΣ≈δΚœΈοΘ§Ζ¥”ΠΈΣFe2++NO=FeΘ®NOΘ©2+Θ§Ι ¥πΑΗΈΣΘΚΈϋ ’Έ¥Ζ¥”ΠΒΡNOΘΜ
Θ®4Θ©CuNH4Θ®OHΘ©CrO4‘Ύ295ΓφΖ÷Ϋβ…ζ≥…Μν–‘―«ΗθΥαΆ≠¥ΏΜ·ΦΝΓΔ“Μ÷÷ΈόΕΨΤχΧεΦΑΥ°Θ§≤ζΈο÷–ΈόΕΨΤχΧεΈΣΒΣΤχΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣ2CuΘ®OHΘ©NH4CrO4 Cr2O3?2CuO+N2Γϋ+5H2OΘ§
Cr2O3?2CuO+N2Γϋ+5H2OΘ§
Ι ¥πΑΗΈΣΘΚ2CuΘ®OHΘ©NH4CrO4 Cr2O3?2CuO+N2Γϋ+5H2OΘΜ
Cr2O3?2CuO+N2Γϋ+5H2OΘΜ
Θ®5Θ©ΗυΨίΖ¥”Π6NO+4NH3®T5N2+6H2OΦΤΥψΘ§‘Ύ“ΜΕ®ΧθΦΰœ¬ΤχΧεΒΡΧεΜΐ÷°±»Β»”ΎΈο÷ ΒΡΝΩ÷°±»Θ§
6NO+4NH3®T5N2+6H2O
6ml 5ml
V 2016ml
V=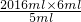 =2419.2mlΘ§‘ρNOΒΡΉΣΜ·¬ ΈΣ
=2419.2mlΘ§‘ρNOΒΡΉΣΜ·¬ ΈΣ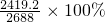 =90%Θ§
=90%Θ§
Ι ¥πΑΗΈΣΘΚ90%Θ°
Ζ÷ΈωΘΚΘ®1Θ©‘ΎΦ”»»ΧθΦΰœ¬Θ§ Β―ι “≥Θ”Ο¬»Μ·οßΚΆ«β―θΜ·ΗΤΖ¥”Π÷Τ±ΗΑ±ΤχΘ§Ά§ ±…ζ≤欻̷ΗΤΚΆΥ°Θ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘΜ
Θ®2Θ©ΗυΨίΆ≠ΚΆœΓœθΥαΖ¥”Π…ζ≤ζœθΥαΆ≠ΚΆ“Μ―θΜ·ΒΣΫαΚœΖ¥”ΠΉώ―≠ΒγΚ… ΊΚψΓΔΒγΉ” ΊΚψ“‘ΦΑ÷ ΝΩ ΊΚψά¥ ι–¥άκΉ”ΖΫ≥Χ ΫΘ§”ΟΩ…≥ιΕ·ΒΡΆ≠ΥΩ“Ή”ΎΩΊ÷ΤΖ¥”ΠΘ§≤ΌΉςΖΫ±ψΘ°
Θ®3Θ©ΝρΥα―«ΧζΈϋ ’NOΘ§…ζ≥…≈δΚœΈοΘΜ
Θ®4Θ©CuNH4Θ®OHΘ©CrO4‘Ύ295ΓφΖ÷Ϋβ…ζ≥…Μν–‘―«ΗθΥαΆ≠¥ΏΜ·ΦΝΓΔ“Μ÷÷ΈόΕΨΤχΧεΦΑΥ°Θ§≤ζΈο÷–ΈόΕΨΤχΧεΈΣΒΣΤχΘ§ΗυΨί÷ ΝΩ ΊΚψ ι–¥Μ·―ßΖΫ≥Χ ΫΘΜ
Θ®5Θ©ΗυΨίΖ¥”Π6NO+4NH3®T5N2+6H2OΦΤΥψΉΣΜ·¬ Θ°
ΒψΤάΘΚ±ΨΧβΩΦ≤ιΑ±ΤχΒΡ÷Τ»ΓΚΆ–‘÷ Β»÷Σ ΕΘ§ΧβΡΩΫœΈΣΉέΚœΘ§ΨΏ”–“ΜΕ®Ρ―Ε»Θ§±ΨΧβ÷–ΉΔ“β…ΌΝΩΑ±Τχ÷Τ»ΓΒΡΖΫΖ®“‘ΦΑNOΈ≤ΤχΒΡΈϋ ’Β»Έ ΧβΘ§’β‘ΎΩΈ±Ψ÷– «≤ΜΕύΦϊΒΡΘ°
NH4Cl+CaΘ®OHΘ©2
 CaCl2+2NH3Γϋ+2H2OΘ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘ§
CaCl2+2NH3Γϋ+2H2OΘ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘ§Ι ¥πΑΗΈΣΘΚ2NH4Cl+CaΘ®OHΘ©2
 CaCl2+2NH3Γϋ+2H2OΘΜ≈®Α±Υ°ΓΔΦν ·Μ“ΘΜ
CaCl2+2NH3Γϋ+2H2OΘΜ≈®Α±Υ°ΓΔΦν ·Μ“ΘΜΘ®2Θ©Ά≠ΚΆœΓœθΥαΖ¥”Π…ζ≤ζœθΥαΆ≠ΚΆ“Μ―θΜ·ΒΣΘ§άκΉ”ΖΫ≥Χ ΫΈΣ3Cu+8H++2NO3-=3Cu2++2NOΓϋ+4H2OΘ§”ΟΩ…≥ιΕ·ΒΡΆ≠ΥΩ“Ή”ΎΩΊ÷ΤΖ¥”ΠΘ§≤ΌΉςΖΫ±ψ≤ΔΡήΫΎ‘Φ“©ΤΖΘ§
Ι ¥πΑΗΈΣΘΚ3Cu+8H++2NO3-=3Cu2++2NOΓϋ+4H2OΘΜΖ¥”ΠΩ…“‘Υφ ±ΙΊΆΘΓΔ≤ΌΉςΖΫ±ψΓΔΖ¥Η¥ Ι”ΟΓΔΫΎ‘Φ“©ΤΖΘΜ
Θ®3Θ©“Μ―θΜ·ΒΣ”κΕΰΦέΧζ–Έ≥…≈δΈΜΦϋΘ§»ή“Κ÷–Β≠¬Χ…ΪΒΡΕΰΦέΧζ±δ≥…ΉΊ…ΪΒΡ“Μ―θΜ·ΒΣ≈δΚœΈοΘ§Ζ¥”ΠΈΣFe2++NO=FeΘ®NOΘ©2+Θ§Ι ¥πΑΗΈΣΘΚΈϋ ’Έ¥Ζ¥”ΠΒΡNOΘΜ
Θ®4Θ©CuNH4Θ®OHΘ©CrO4‘Ύ295ΓφΖ÷Ϋβ…ζ≥…Μν–‘―«ΗθΥαΆ≠¥ΏΜ·ΦΝΓΔ“Μ÷÷ΈόΕΨΤχΧεΦΑΥ°Θ§≤ζΈο÷–ΈόΕΨΤχΧεΈΣΒΣΤχΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣ2CuΘ®OHΘ©NH4CrO4
 Cr2O3?2CuO+N2Γϋ+5H2OΘ§
Cr2O3?2CuO+N2Γϋ+5H2OΘ§Ι ¥πΑΗΈΣΘΚ2CuΘ®OHΘ©NH4CrO4
 Cr2O3?2CuO+N2Γϋ+5H2OΘΜ
Cr2O3?2CuO+N2Γϋ+5H2OΘΜΘ®5Θ©ΗυΨίΖ¥”Π6NO+4NH3®T5N2+6H2OΦΤΥψΘ§‘Ύ“ΜΕ®ΧθΦΰœ¬ΤχΧεΒΡΧεΜΐ÷°±»Β»”ΎΈο÷ ΒΡΝΩ÷°±»Θ§
6NO+4NH3®T5N2+6H2O
6ml 5ml
V 2016ml
V=
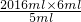 =2419.2mlΘ§‘ρNOΒΡΉΣΜ·¬ ΈΣ
=2419.2mlΘ§‘ρNOΒΡΉΣΜ·¬ ΈΣ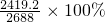 =90%Θ§
=90%Θ§Ι ¥πΑΗΈΣΘΚ90%Θ°
Ζ÷ΈωΘΚΘ®1Θ©‘ΎΦ”»»ΧθΦΰœ¬Θ§ Β―ι “≥Θ”Ο¬»Μ·οßΚΆ«β―θΜ·ΗΤΖ¥”Π÷Τ±ΗΑ±ΤχΘ§Ά§ ±…ζ≤欻̷ΗΤΚΆΥ°Θ§ Β―ι “÷Τ±Η…ΌΝΩΑ±Τχ ±Ω…ΗυΨίΑ±Υ°ΒΡ“ΉΜ”ΖΔ–‘ΚΆ―θΜ·ΗΤΒΡΈϋΥ°–‘ά¥÷Τ»ΓΘΜ
Θ®2Θ©ΗυΨίΆ≠ΚΆœΓœθΥαΖ¥”Π…ζ≤ζœθΥαΆ≠ΚΆ“Μ―θΜ·ΒΣΫαΚœΖ¥”ΠΉώ―≠ΒγΚ… ΊΚψΓΔΒγΉ” ΊΚψ“‘ΦΑ÷ ΝΩ ΊΚψά¥ ι–¥άκΉ”ΖΫ≥Χ ΫΘ§”ΟΩ…≥ιΕ·ΒΡΆ≠ΥΩ“Ή”ΎΩΊ÷ΤΖ¥”ΠΘ§≤ΌΉςΖΫ±ψΘ°
Θ®3Θ©ΝρΥα―«ΧζΈϋ ’NOΘ§…ζ≥…≈δΚœΈοΘΜ
Θ®4Θ©CuNH4Θ®OHΘ©CrO4‘Ύ295ΓφΖ÷Ϋβ…ζ≥…Μν–‘―«ΗθΥαΆ≠¥ΏΜ·ΦΝΓΔ“Μ÷÷ΈόΕΨΤχΧεΦΑΥ°Θ§≤ζΈο÷–ΈόΕΨΤχΧεΈΣΒΣΤχΘ§ΗυΨί÷ ΝΩ ΊΚψ ι–¥Μ·―ßΖΫ≥Χ ΫΘΜ
Θ®5Θ©ΗυΨίΖ¥”Π6NO+4NH3®T5N2+6H2OΦΤΥψΉΣΜ·¬ Θ°
ΒψΤάΘΚ±ΨΧβΩΦ≤ιΑ±ΤχΒΡ÷Τ»ΓΚΆ–‘÷ Β»÷Σ ΕΘ§ΧβΡΩΫœΈΣΉέΚœΘ§ΨΏ”–“ΜΕ®Ρ―Ε»Θ§±ΨΧβ÷–ΉΔ“β…ΌΝΩΑ±Τχ÷Τ»ΓΒΡΖΫΖ®“‘ΦΑNOΈ≤ΤχΒΡΈϋ ’Β»Έ ΧβΘ§’β‘ΎΩΈ±Ψ÷– «≤ΜΕύΦϊΒΡΘ°

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ