��Ŀ����
�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
CO2(g) +3H2(g) ��CH3OH(g)+H2O(g) ��H1
2CO (g) +O2(g) ��2CO2(g) ��H2
2H2(g)+O2(g) ��2H2O(g) ��H3
��CO(g) + 2H2(g)  CH3OH(g)���ġ�H�� ��
CH3OH(g)���ġ�H�� ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ��CO(g)+2H2(g) CH3OH(g) �������������䣬��300���500��ʱ�����ʵ���n(CH3OH) �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
CH3OH(g) �������������䣬��300���500��ʱ�����ʵ���n(CH3OH) �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
| A����������� |
| B�������¶� |
| C�������¶� |
| D��ʹ�ú��ʵĴ��� |
��4��CH4��H2O�ڴ������淢����ӦCH4+H2O
 CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O(g)��5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O(g)��5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣�����5���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵ� ��ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��B���ĵ缫��ӦʽΪ ��
�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������·��ת��1mole- ʱ��ʵ�������ĵļ״��������������ϴ���ԭ���� ��
��6��25��ʱ������Ƶ�Ksp=4.0��10-8,̼��Ƶ�Ksp=2.5��10-9����20ml̼��Ƶı�����Һ����μ���8.0��10-4 mol��L-1�IJ������Һ20ml���ܷ�������� ����ܡ�����
��1����H1+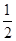 ��H2 ��
��H2 ��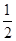 ��H3 ��2���� ��3��ABE ��4��6.75
��H3 ��2���� ��3��ABE ��4��6.75
��5����CH3OH + 3O2- -6e-= CO2+ 2H2O���ڼ״�����ȫ������������C��CO �� �������ת���ʴﲻ��100% ���������𰸶����� ��6����
���������������1�����ݸ�˹���ɼ��㣺�ܷ�ӦΪ��+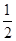 �ڣ�
�ڣ�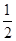 �ۣ��ܷ�Ӧ�ġ�Hͬ���仯��
�ۣ��ܷ�Ӧ�ġ�Hͬ���仯��
��2����300�浽500�棬n(CH3OH)��С��˵�����»�ѧƽ�������ƶ�����Ӧ�ġ�H��0��
��3����Ӧ�����������С�ķ��ȷ�Ӧ����״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��ѹ��С��������������¶ȣ����״��ӻ����ϵ�з�������ȷ���ʹ��Ӧƽ�������ƶ���
��4����Ӧ��ƽ�ⳣ��������������(1L���������ʵ����ʵ�����������Ũ��)��
CH4 + H2O CO + 3H2
CO + 3H2
ʼ�� 1 1 0 0
�䣺0.5 0.5 0.5 1.5
ƽ��0.5 0.5 0.5 1.5
K=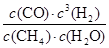 =
=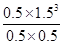 =6.75��
=6.75��
��5����B����CH3OH����ĸ����������ʹ�����O2-��Ӧ��ʧȥ��������CO2��H2O����ʵ�������ĵļ״��������������ϴ��ԭ��������ӷ�Ӧ���������״�û�вμ�ȫ����Ӧ����ת���̶��������״���̼Ԫ�ؿ��Ա仯Ϊ0��+2��+4�ۣ��������ɵ���̼��COʱת�Ƶĵ����٣�������ת����������ѧ�ܳ��˱�Ϊ���ܣ������������ܡ�
��6��̼��Ƶı�����Һ��c(Ca2+)=c(CO32-)=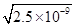 mol��L-1=5��10-5 mol��L-1��ϡ��1�����룻����Һ�������ز���Ӧ�����c(C2O42-)=4.0��10-4 mol��L-1����ʱc(Ca2+)��c(C2O42-)=4.0��10-4��2.5��10-5=1.0��10-8��4.0��10-8�������г�����
mol��L-1=5��10-5 mol��L-1��ϡ��1�����룻����Һ�������ز���Ӧ�����c(C2O42-)=4.0��10-4 mol��L-1����ʱc(Ca2+)��c(C2O42-)=4.0��10-4��2.5��10-5=1.0��10-8��4.0��10-8�������г�����
���㣺��ѧ��Ӧԭ���ۺϡ���ѧ��Ӧ�ȡ���Ӧ��ת��������ƽ�ⳣ�����㡢��������������Եļ��㡣

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�������һЩ������ȼ����(kJ/mol)���ݣ��ش��������⣺
| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891��0 | ������ | 2 878��0 |
| ���� | 1560��8 | �춡�� | 2 869��6 |
| ���� | 2 221��5 | 2-������ | 3 531��3 |
��1����֪�����ʵ�����Խ��Խ���ȶ������ݱ��е����ݣ��Ƚ������顢�춡������ȶ��ԣ�������______�춡��(�>������������<��)��
��2��д������ȼ�յ��Ȼ�ѧ����ʽ��________________________
��3����ͬ������������̼����������Խ��ȼ�շų�������______(�Խ�ࡱ����Խ�١�����ͬ��)��
����β���е�NOx�Ǵ�����Ⱦ��֮һ����ѧ�����ڳ����ø���ѧ�ķ�����NOxת���������ʣ��Ӷ���������β����Ⱦ��
��1��ѹ����Ȼ����CNG���������ŵ�֮һ�����ô������ܹ���NOxת�������CO2��N2��
��CH4(g)��4NO2(g)  4NO(g)��CO2(g)��2H2O(g) ��H1��0
4NO(g)��CO2(g)��2H2O(g) ��H1��0
��CH4(g)��4NO(g) 2N2(g)��CO2(g)��2H2O(g) ��H2��0
2N2(g)��CO2(g)��2H2O(g) ��H2��0
��CH4(g) ��2NO2(g) N2(g) ��CO2(g) ��2H2O(g) ��H3�� �����á�H1�͡�H2��ʾ��
N2(g) ��CO2(g) ��2H2O(g) ��H3�� �����á�H1�͡�H2��ʾ��
��2���ں�ѹ�£���CH4(g)��NO2(g)�����ܱ������з�����ѧ��Ӧ�ۣ��ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��NO2��ƽ��ת���ʼ��±���
| Ͷ�ϱ�[n(NO2) / n(CH4)] | 400 K | 500 K | 600 K |
| 1 | 60% | 43% | 28% |
| 2 | 45% | 33% | 20% |
��д���÷�Ӧƽ�ⳣ���ı���ʽK= ��
�����¶Ȳ��䣬���[n(NO2) / n(CH4)]Ͷ�ϱȣ���K�� �������������С�����䡱����
��400 Kʱ����Ͷ�ϱ�Ϊ1��NO2��CH4�Ļ�����干0.04 mol������һװ�д����������У���ַ�Ӧ��ƽ��ʱNO2��������� ��
��3�������Զ��������NOx����������̬�����ǵĹ���ԭ��ʾ��ͼ��ͼ1


ͼ1 ͼ2
��NiO�缫��NO�����ĵ缫��Ӧʽ�� ��
���ռ�ij����β��������NOx�ĺ���Ϊ1.12%����������������ü��齫����ȫת��Ϊ�����壬����1��104L����״���£���β����Ҫ����30g����β����V(NO)�UV(NO2)=
��4�����ݻ���ͬ�������ܱ������� (װ�е�����ij�ִ���) �ȸ�ͨ�������CH4��Ȼ���ٷֱ���������NO��NO2���ڲ�ͬ�¶��£�ͬʱ�ֱ����ڢ�������Ӧ������t��ʱ�ⶨ����NOxת���ʣ����ͼ����ͼ2��ʾ��
�ٴ�ͼ�п��Եó��Ľ�����
����һ����ͬ�¶���NOת��Ч�ʱ�NO2�ĵ�
���۶�����250��-450��ʱ��NOxת�������¶����߶�����450��-600��ʱNOxת�������¶����߶���С
���۶���ԭ����
��������NO2��CH4��Ӧ�У����NO2ת���ʵĴ�ʩ��_________��(����)
A�����ø�Ч���� B�������¶� C.�����H2O(g) D������ѹǿ
E������ԭ�����ı���� F����СͶ�ϱ�[n(NO2) / n(CH4)]
�Ժ������ʵ��о����������ż�Ϊ��Ҫ�����塣
��1��N2��O2��H2�֮����Է������Ϸ�Ӧ����֪��Ӧ���Ȼ�ѧ����ʽ���£�
N2(g)+O2(g)=2NO(g) 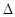 H=+180��5kJ��mol-1��
H=+180��5kJ��mol-1��
2H2(g)+O2(g)=2H2O(g) 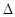 H =-483��6 kJ��mol-1��
H =-483��6 kJ��mol-1��
N2(g)+3H2(g)=2NH3(g) 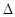 H =-92��4 kJ��mol-1��
H =-92��4 kJ��mol-1��
�Ĵ�������Ӧ���Ȼ�ѧ����ʽΪ ��
��2������β��������һ����Ӧԭ��Ϊ��2NO(g)+2CO(g) N2(g)+2CO2(g)
N2(g)+2CO2(g) 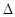 H<0��
H<0��
һ���¶��£���2��8mol NO��2��4mol COͨ��̶��ݻ�Ϊ2L���ܱ������У���Ӧ�����в������ʵ����ʵ����仯��ͼ��ʾ��
��NO��ƽ��ת����Ϊ ��0~20minƽ����Ӧ����v(NO)Ϊ ��25minʱ�������ַ�Ӧ�¶Ȳ��䣬���������г���CO��N2��0��8 mol����ѧƽ�⽫ �ƶ�(����������ҡ�����)��
����ֻ�ı�ijһ��Ӧ����X����Ӧ��ԭƽ��I�ﵽ��ƽ��II������Y�ı仯��������ͼ��ʾ������˵����ȷ���� (����ĸ����)��
|
 ��3��ij��ѧС���������N2��H2Ϊ�缫��Ӧ���HCl��NH4ClΪ�������Һ�Ƴ�ȼ�ϵ�أ���õ�ص�������ӦʽΪ ������������Һ��������䣬����˵����ȷ���� (����ĸ����)��
��3��ij��ѧС���������N2��H2Ϊ�缫��Ӧ���HCl��NH4ClΪ�������Һ�Ƴ�ȼ�ϵ�أ���õ�ص�������ӦʽΪ ������������Һ��������䣬����˵����ȷ���� (����ĸ����)��a���ŵ�����У��������Һ��pH���ֲ���
b����Һ�е�NH4ClŨ������Cl-����Ũ�Ȳ���
c��ÿת��6��02
 1023�����ӣ����б�״����11��2L�缫��Ӧ�ﱻ����
1023�����ӣ����б�״����11��2L�缫��Ӧ�ﱻ����d��Ϊ���ַŵ�Ч�������ʹ��һ��ʱ��������������Һ
 H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣ 2NH3����֪,�ڳ�����,1 g H2��ȫת��ΪNH3,�ų�������Ϊ15.4 kJ��
2NH3����֪,�ڳ�����,1 g H2��ȫת��ΪNH3,�ų�������Ϊ15.4 kJ�� 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
 N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol