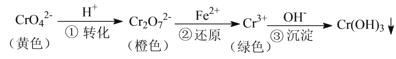��Ŀ����
����Ŀ��ij��ѧ��ȤС��������̼���Ͻ�ĺ�̼�����������������ʵ�鷽����
������̼���Ͻ��ĩ��Ũ������һ�������·�Ӧ��������װ��![]() �ӳ�װ��ʡ��
�ӳ�װ��ʡ��![]() �ȿɼ�������г�
�ȿɼ�������г�![]() ��������ijɷ֣��ֿ�ͨ������
��������ijɷ֣��ֿ�ͨ������![]() ������������Ͻ�ĺ�̼����
������������Ͻ�ĺ�̼����

��1���Ͻ���Ũ������һ������������![]() �ķ�Ӧ�У�����ԭ��������ʱ____���ѧʽ����
�ķ�Ӧ�У�����ԭ��������ʱ____���ѧʽ����
��2��Ϊʵ��ʵ��Ŀ�ģ�����������ȷ������˳��![]() ����������
����������![]() ����������____��____��______����a����b����_____��_______����k����l����
����������____��____��______����a����b����_____��_______����k����l����
��3��װ��E����װҩƷΪ ______ ![]() ����ĸ���
����ĸ���![]() ���ڱ�ʵ���е������� ______ ��
���ڱ�ʵ���е������� ______ ��
A ��ʯ�� B ��ˮ�Ȼ��� C Ũ����
��4�����ܹ۲쵽 ______ ��������֤��ԭ���������һ������![]() ��
��
��5��ʵ������ղ����ĺ�̼��ƫ�ͣ�ijͬѧ�²����������п��ܺ���CO����G������һ��װ�ü�����֤���IJ²⣬���Ҫ˵����֤����²�����װ�ü�ҩƷ�;���ʵ������ ______ ��
������ȡ��ĩ״��Ʒ![]() ������ijŨ�ȵ�ϡ����100mL����ַ�Ӧ���ռ�����״��������
������ijŨ�ȵ�ϡ����100mL����ַ�Ӧ���ռ�����״��������![]() ��Ȼ���������Ʒ�м���ͬŨ�ȵ�ϡ����100mL����ַ�Ӧ�����ռ�����״��������1.12L����Ͻ���̼����������Ϊ ______��������λ��Ч���֣���
��Ȼ���������Ʒ�м���ͬŨ�ȵ�ϡ����100mL����ַ�Ӧ�����ռ�����״��������1.12L����Ͻ���̼����������Ϊ ______��������λ��Ч���֣���
�������������շ�ʹ�Ͻ��е�̼ת��Ϊ������̼���Ͻ����������Ӷ����㺬̼�����������ǽ�һ��������Ʒ���պ����������������ˣ���ԭ���� ______ ��
���𰸡�C��Fe k l d c e f gh ji A ���ն�����̼ Fװ���к�ɫ��ĩ���ɫ��װ��G�еİ�ɫ��ĩ����ɫ Gװ�ú��һ��װ�г���ʯ��ˮ��ϴ��ƿ������ʯ��ˮ�����˵�������������CO������ʯ��ˮ�������˵����������в�����CO 1.18% ����ʱ����������Ӧ���������������ʹʣ������������ӣ�̼��������Ӧ�ų��Ķ�����̼��ʹ����������С�����Ͻ��к�����̼��
��������
������1���Ͻ��Ǻ�̼���Ͻ���Ũ������̼�����������ڼ��������·�����Ӧ����Ũ����������̼����ԭ��������Ϊ������̼��������ԭ��������Ϊ����,�ʴ�Ϊ��C��Fe��
��2����̼���Ͻ��ĩ��Ũ������һ�������·�Ӧ���ɲ����ж�����������̼��ˮ�������淴Ӧ����Ũ�����ϡ������ϡ���ᷴӦ��������������������Ҫ�Ƕ�����̼����������������ˮ������������ʵ�������������װ�ã�Ӧ�ѵõ��Ļ��������ͨ��װ��G�������ɵ�ˮ����������ͭ����֤��ˮ���ɣ�ͨ��װ��B��Ʒ����ɫ�����������Ĵ��ڣ�ͨ��װ��C�и��������Һ��ȥ���������ٺ���ʵ��Ӱ�죬ͨ��װ��A��Ũ�����ȥ�����е�ˮ������ͨ��װ��E�м�ʯ�����������ж�����̼�����������仯�������ɵĶ�����̼���壬��ͨ��װ��F��G�����Ƿ����������ɣ����Ϊʵ��ʵ��Ŀ�ģ�����������ȷ������˳��Ϊ��kl��dc��ef��ab��gh��ji��kl��
��3����������������֪װ��E����װҩƷ��ʯ�����ն�����̼���壬���ڳ�������ǰ�������仯���������̼�������ʴ�Ϊ��A�����ն�����̼��
��4����֤��������������������װ��F������ͭ��������ԭΪ��ɫͭ��װ��G����ˮ����ͭ����ɫ������ˮ��֤����������ԭ����ͭ���ɵ�ˮ�������ʴ�Ϊ��Fװ���к�ɫ��ĩ���ɫ��װ��G�еİ�ɫ��ĩ����ɫ��
��5����֤����������Ƿ���ܺ���CO������Fװ���У�һ����̼��ԭ����ͭ���ɶ�����̼����Gװ�ú�����װ�г���ʯ��ˮ��ϴ��ƿ���м��飬�������֤����һ����̼�������У��ʴ�Ϊ��Gװ�ú��һ��װ�г���ʯ��ˮ��ϴ��ƿ������ʯ��ˮ�����˵�������������CO������ʯ��ˮ�������˵����������в�����CO��
������Fe��ȫ��Ӧ�����������![]() ���������������ʵ���
���������������ʵ���![]() �����ݵ���ת���غ㣬
�����ݵ���ת���غ㣬![]() ����
����![]() ��
��![]() ��̼������
��̼������![]() ���ʸ�������Ʒ��̼����������
���ʸ�������Ʒ��̼����������![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
����������ʱ������������Ӧ���������������ʹʣ������������ӣ�̼��������Ӧ�ų�������̼��ʹʣ������������٣����Ͻ��к��н϶������ֻ��������̼����˽�һ��������Ʒ���պ����������������ˣ��ʴ�Ϊ������ʱ����������Ӧ���������������ʹʣ������������ӣ�̼��������Ӧ�ų��Ķ�����̼��ʹ����������С�����Ͻ��к�����̼�١�

����Ŀ������±��ش�������������Ϊ�����µ�������:
�� | ���볣����Ka�� | �� | ���볣����Ka�� | �� | ���볣����Ka�� | �� | ���볣����Ka�� |
CH3COOH | 1.8��10-5 | H2CO3 | K1=4.4��10-7 | H2C2O4 | K1=5.4��10-2 | H2S | K1=1.3��10-7 |
HClO | 3��10-8 | K2=4.7��10-11 | K2=5.4��10-5 | K2=7.1��10-15 |
��ش���������:
��1�� ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��HC2O4-��ClO-��S2-�н��H+��������������_________��
��2�� 0.1mo1/L��H2C2O4��Һ��0.1mo1/L��KOH����Һ�������Ϻ�������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��
��3��pH��ͬ��NaC1O��CH3COOK��Һ�У�[c(Na+)-c(C1O-)]______[c(K+)-c(CH3COO-)]������>������<������=���� ��
��4�� ��0.1mo1/LCH3COOH ��Һ�еμ�NaOH ��Һ��c(CH3COOH): c(CH3COO-)=5:9����ʱ��ҺpH=_________��