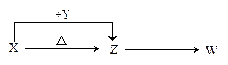��Ŀ����
��֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F��ʹ��ɫʪ��ʯ����ֽ����ɫ������֮���ܷ������·�Ӧ��
�� A+H2O �� B+C �� C+F �� D �� D+NaOH �� F+E+H2O
��1��д�����ǵĻ�ѧʽ��D ��F ��
��2��д���ٷ�Ӧ�Ļ�ѧ����ʽ�� ��
�˷�Ӧ���������� ����ԭ���� ��
��3��д����Ӧ�۵����ӷ���ʽ�� ��
��4����ҵ����C�Ĺ�����������һ����Ӧ����F������������B��H2O��д���ò���Ӧ�Ļ�ѧ����ʽ�� ��
�� A+H2O �� B+C �� C+F �� D �� D+NaOH �� F+E+H2O
��1��д�����ǵĻ�ѧʽ��D ��F ��
��2��д���ٷ�Ӧ�Ļ�ѧ����ʽ�� ��
�˷�Ӧ���������� ����ԭ���� ��
��3��д����Ӧ�۵����ӷ���ʽ�� ��
��4����ҵ����C�Ĺ�����������һ����Ӧ����F������������B��H2O��д���ò���Ӧ�Ļ�ѧ����ʽ�� ��
��1��NH4NO3��2�֣��� NH3
��2��3NO2+H2O �� 2HNO3 +NO �� NO2�� NO2
��3��NH4�� +OH��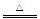 NH3��+H2O
NH3��+H2O
��4��4NH3+5O2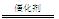 4NO+6H2O
4NO+6H2O
��2��3NO2+H2O �� 2HNO3 +NO �� NO2�� NO2
��3��NH4�� +OH��
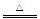 NH3��+H2O
NH3��+H2O ��4��4NH3+5O2
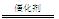 4NO+6H2O
4NO+6H2O ��

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��
���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��
 ____________��
____________��