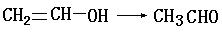��Ŀ����
��ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯 ��
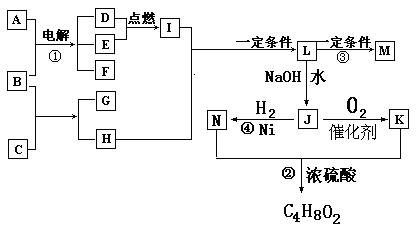
�� ���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��
���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��

��֪��
(a)A��B��C��D�Ƿǽ������ʣ�����B��C��D�ڳ��³�ѹ�������壮
(b)��Ӧ�١����ǻ��������е���Ҫ��Ӧ��
(c)������E���γ��������Ⱦ��֮һ��������K�dz��õĵ��ʣ�
(d)������L����Ư���ԣ�����Cl2��NaOH��Һ��Ӧ���Ƶã�
(e)������J������Ԫ����ɣ�����Է�������Ϊ32.
�밴Ҫ����գ�
(1)��Ӧ�۵Ļ�ѧ����ʽ_____________________��
(2)C�Ľṹʽ__________________________��
HΪ���Σ���H�Ļ�ѧʽΪ________ ____________��
____________��
(3)L����Һ�뻯����E��Ӧ�����ӷ���ʽ�� ______________.
(4)������J�Ļ�ѧʽ____________________��
 ��
�� ���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��
���ת����ϵͼ(�йط�Ӧ�����������ɵ�H2O����ȥ)��
��֪��
(a)A��B��C��D�Ƿǽ������ʣ�����B��C��D�ڳ��³�ѹ�������壮
(b)��Ӧ�١����ǻ��������е���Ҫ��Ӧ��
(c)������E���γ��������Ⱦ��֮һ��������K�dz��õĵ��ʣ�
(d)������L����Ư���ԣ�����Cl2��NaOH��Һ��Ӧ���Ƶã�
(e)������J������Ԫ����ɣ�����Է�������Ϊ32.
�밴Ҫ����գ�
(1)��Ӧ�۵Ļ�ѧ����ʽ_____________________��
(2)C�Ľṹʽ__________________________��
HΪ���Σ���H�Ļ�ѧʽΪ________
 ____________��
____________��(3)L����Һ�뻯����E��Ӧ�����ӷ���ʽ�� ______________.
(4)������J�Ļ�ѧʽ____________________��
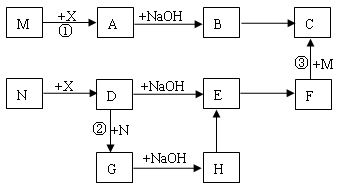
(1)2NH3��H2SO4===(NH4)2SO4��2�֣�
(2)N��N��2�֣� ��(NH4)2SO3��2�֣�
(3)ClO����SO2��2OH��===Cl����SO��H2O��2�֣�
(4)N2H4��2�֣�
(2)N��N��2�֣� ��(NH4)2SO3��2�֣�
(3)ClO����SO2��2OH��===Cl����SO��H2O��2�֣�
(4)N2H4��2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2������X�����Ԫ��ԭ�ӽṹ��ͼ �� ��
2������X�����Ԫ��ԭ�ӽṹ��ͼ �� ��