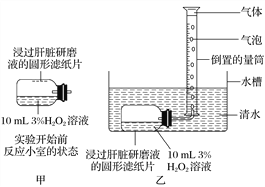
��Ŀ����
����Ŀ����ҩҩ����˪����Ҫ�ɷ�ΪAs2O3������ˮ�������������Ƽ���Ѫ������ҵ���ú����飨As2S3)�ķ���������˪�Ĺ���������ͼ��ʾ��
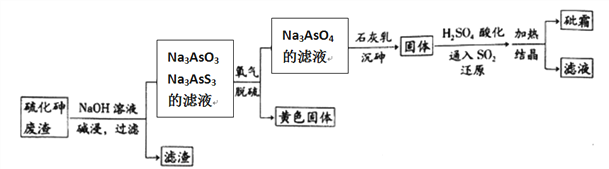
��1����������Ԫ�ػ��ϼ�Ϊ__________����������ˮ�����������������Ԫ�ؼ�̬���䣬��������Ҫ��Ӧ�����ӷ���ʽΪ________________________��
��2��������������б�������Ԫ����____________��
��3����ԭ������H3AsO4ת��ΪH3AsO3����Ӧ�Ļ�ѧ����ʽ��____________________��
��4��������������һ���¶��½���Ԫ��ת��ΪCa5(AsO4)3OH�����Ĺ��̣���Ҫ��Ӧ�У�
a.Ca(OH)2(S)![]() Ca2+(aq) +2OH-(aq) Ksp=10-7
Ca2+(aq) +2OH-(aq) Ksp=10-7
b.Ca5(AsO4)3OH (S)![]() 5Ca2+(aq)+OH-(aq)+3AsO43-(aq) Ksp=10-40
5Ca2+(aq)+OH-(aq)+3AsO43-(aq) Ksp=10-40
����ʯ�������Һ��c(OH-) =0.01mol L-1����ʱ��Һ��c(AsO43-) =________________��(��֪�� ![]() =2. 15)
=2. 15)
��5����ԭ�������Һ��H3AsO3�ֽ�ΪAs2O3���ᾧ�õ���As2O3��As2O3�ڲ�ͬ�¶ȺͲ�ͬŨ�������е��ܽ�ȶ�(S)��������ͼ��ʾ��
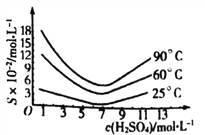
Ϊ����ߴ�As2O3�ij����ʣ����ᾧ�����̽��еIJ���������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ��ڴ˹�����Ӧ���Ƶ�����Ϊ_________________��
��6���ڹ�ҵ�����У����һ��������Һ��ѭ��ʹ�ã���Ŀ����____________________��
��7����Ԫ�ع㷺��������Ȼ�磬�����仯���ﱻ������ũҩ�����ݼ���ɱ����ȡ�
����ij�����������As2O3��As2O5������As2O5���ȶ��Բ����ͼ1д��As2O5�ֽ�ΪAs2O3���Ȼ�ѧ����ʽ___________________________________________��
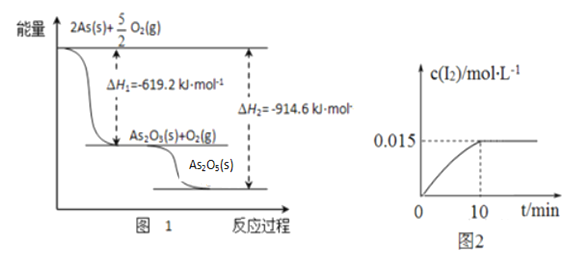
�������ƾ��������ԣ�298 Kʱ����100 mL�ձ��м���10 mL 0.1 mol/L Na3AsO4��Һ��20 mL 0.1 mol/L KI��Һ��20 mL 0.05 mol/L������Һ���������з�Ӧ��AsO43-(��ɫ)+2I-+2H+![]() AsO33-(��ɫ)+I2(dz��ɫ)+H2O �������Һ��c(I2)��ʱ��(t)�Ĺ�ϵ��ͼ2��ʾ����Һ����仯���Բ��ƣ���������������������淴Ӧ�ﵽƽ��״̬����_______������ĸ���ţ���
AsO33-(��ɫ)+I2(dz��ɫ)+H2O �������Һ��c(I2)��ʱ��(t)�Ĺ�ϵ��ͼ2��ʾ����Һ����仯���Բ��ƣ���������������������淴Ӧ�ﵽƽ��״̬����_______������ĸ���ţ���
a����Һ��ɫ���ֲ��ٱ仯 b��c(AsO33-)+c(AsO43-)���ٱ仯
c��AsO43-���������ʵ���I2���������� d�� ![]() ���ֲ��ٱ仯
���ֲ��ٱ仯
���𰸡� +3 As2S3+6OH- =AsO33-+AsS33-+3H2O As �� S SO2+H3AsO4+H2O=H3AsO3+H2SO4 2.15��10-8mol��L-1 ������Ũ��ԼΪ7 mol��L-1����ȴ���¶�Ϊ25�� �����Ļ����� As2O5(s)��As2O3(s)+O2(g ) ��H��+295.4 kJ/mol ac
��������(1).SԪ�صõ�����ʱ����-2�ۣ����ɼ��������Na3AsO3Ҳ�ɿ�����As��+3�ۣ���Ϊ�����������Ԫ�ؼ�̬���䣬������ͼ��֪����Na3AsO3��Na3AsS3�����ӷ���ʽΪ��As2S3+6OH- =AsO33-+AsS33-+3H2O��
(2).����������As������Ϊ+5�ۣ�SԪ�ر�����ΪS���ʣ����Ա�������Ԫ��ΪAs��S��
(3).��H3AsO4��SO2��ϣ�����H3AsO3��SO2���������䷴Ӧ����ʽΪ��SO2+H3AsO4+H2O=H3AsO3+H2SO4
(4).�ɷ�Ӧa��c(OH-) =0.01mol L-1��![]() ���ɷ�Ӧb�ɵ�
���ɷ�Ӧb�ɵ�![]() ��
��
(5).��ͼ2��֪��As2O3��25��C������Ũ��ԼΪ7mol/Lʱ���ܽ����С���ʡ��ᾧ������Ӧ��������Ũ��Ϊ7mol/L����ȴ���¶�25��C��
��6���ڹ�ҵ�����У����һ��������Һ��ѭ��ʹ�ÿ����As�Ļ����ʣ�
��7����As2O5���ȶ��Բ�ֽ�ΪAs2O3��O2��ͬʱ�ܾ߸�˹���ɿ�֪����H��|��H2|-|��H1|=914.6 kJ/mol-619.2 kJ/mol=+295.4 kJ/mol�����Ը÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ��As2O5(s)��As2O3(s)+O2(g ) ��H��+295.4 kJ/mol��
��a.����Һ��ɫ����ʱ��I2�����������ı䣬��Ӧ�ﵽƽ�⣬a��ȷ��b.����ѧ��������ȣ���ֵ���淴Ӧ�仯���仯��b����c.������������ͬ��˵�����淴Ӧ������ͬ����Ӧ�ﵽƽ�⣬c��ȷ��d.![]() Ϊ��ֵ�����ܷ�ӳ��Ӧ���ʵı仯��d����ѡ��ac��
Ϊ��ֵ�����ܷ�ӳ��Ӧ���ʵı仯��d����ѡ��ac��

����Ŀ���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ�����±�ͼ���ʾ25��ʱ��ϡ��CH3COOH��HClO�������ϡ��Һ����ҺpH���ˮ���ı仯�������£��й�˵����ȷ����
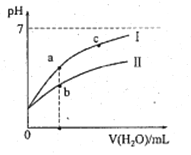
����ĵ���ƽ�ⳣ��(25��) | |
CH3COOH | HClO |
Ka=1.8��10-5 | Ka=3.0��10-8 |
A. ͼ���У�a�������Ũ��>b�������Ũ��
B. ͼ���У�c(H+)��c(R-)��ֵ��a��>c��(HR����CH3COOH��HClO)
C. pH��ͬ��CH3COONa��Һ��NaClO��Һ��Ũ�ȹ�ϵ:c(CH3COOHNa)<c(NaClO)
D. ����ҺŨ����ȣ�CH3COONa��Һ��c(OH-)+c(CH3COO-)>NaClO��Һ��c(OH-)+c(C1O-)