��Ŀ����
����Ŀ��ʵ������Ҫ����250ml 1.6mol��L��1��NaOH��Һ����ش��������⣺
��1����ȡNaOH����______g��
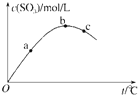
��2�������Ƹ���Һ�Ĺ����У��辭���������ܽ⡢ת����Һ�����ݵȲ���������ͼʾ��Ӧ�IJ����淶��____������ͼʾ���������ȱ�ٵIJ�������������________��__________��

��3�������ƹ����У����������������ȷ�����в���������NaOH��ҺŨ��ƫ�͵���_______��
A. �����õĹ������һ��ʱ�������ܽ����
B. ��Һδ��ȴ�����¼�ת�Ƶ�����ƿ��
C. ����ƿˮϴ��δ����
D. ת����Һʱ������������Һ�γ�ƿ��
E. ����ʱ��С��������ˮ�γ�ƿ��
���𰸡� 16.0 A ϴ�� ҡ�� AD
�����������������������Ҫ��������һ�����ʵ���Ũ����Һ��ʵ�鲽�輰ʵ����������
��1��n=cV=1.6mol/L��0.250L=0.40mol��m=nM=0.40mol��40g/mol=16.0g��
��2������ͼʾ��Ӧ�IJ����淶��A��B��ת��Һ��ʱ���ò�����������C���ʱ�ι�Ӧ��������ƿ���Ϸ������ɲ�������ƿ�ڡ�����ͼʾ���������ȱ�ٵIJ�������������ϴ�ӡ�ҡ�ȡ�
��3��A. ������������ת��Ϊ̼���ƣ�����NaOH��ҺŨ��ƫ�͡�
B. ��Һ��ȴ������ʱ�����С������NaOH��ҺŨ��ƫ�ߡ�
C. ��Ӱ��NaOH��ҺŨ�ȡ�
D. ���ʼ��٣�����NaOH��ҺŨ��ƫ�͡�
E. ��Ӱ��NaOH��ҺŨ�ȡ�
��ѡAD��