��Ŀ����
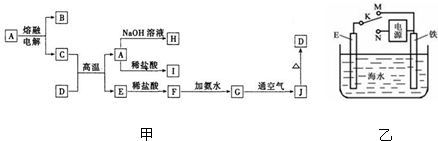
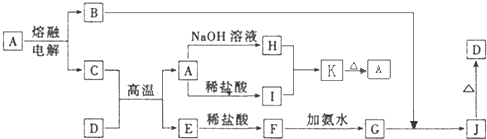
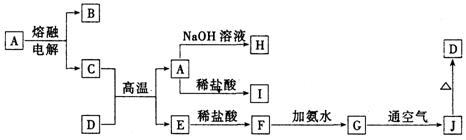
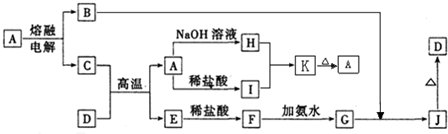
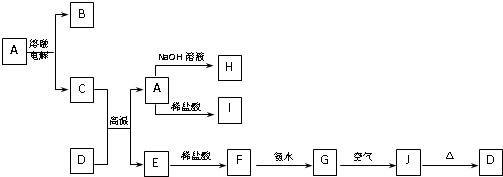
A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
��ش��������⣺
��1��A���ʵ�����Ϊ___________��H��I��Ӧ���������ﻯѧʽΪ ��
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ�������÷�Ӧ��ʵ�������__________ ______��
��3��G��J�Ļ�ѧ����ʽΪ_________________ __________ _____��
��4��A��H�����ӷ���ʽΪ_________ ____ _______��
��5�������ӷ���ʽ��ʾI���������ھ�ˮ��ԭ��_________ _______��
��1�������� Al(OH)3
��2������ ������KClO3������Mg���������ȼ
��3��4Fe(OH)2 + O2 + 2H2O��4Fe(OH)3
��4��Al2O3 + 2OH��+ 3H2O��2[Al(OH)4]��
��5��Al3++ 3H2O![]() Al(OH)3(����)+ 3H+
Al(OH)3(����)+ 3H+
����:
������һ�����͵�����ͼ�ƶ��⡣���������Ԫ�ػ��������֪ʶ�����գ�����Ҫ����ѧ����֪ʶ����ۺ�������������D��һ�ֺ���ɫ�����֪DΪFe2O3����J��Fe(OH)3��G��Fe(OH)2��F��FeCl2��E��Fe����Ͽ�ͼ��Ϣ����֪�������������ʷֱ�Ϊ��A��Al2O3��B��O2��C��Al��H��Na[Al(OH)4]��I��AlCl3��֪����ÿ�����ʣ����⼴������

 Fe��OH��3+3H+
Fe��OH��3+3H+