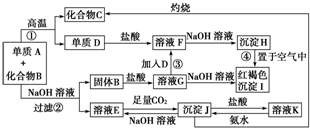
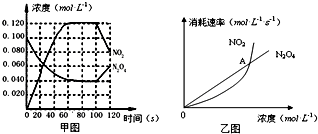
��Ŀ����
�л���A�����ĺ����������ͬ��ͬѹ�£�A�������Ҵ�����������ܶ���2.1.38gA��ȫȼ�պ�����ȼ�յIJ���ͨ����ʯ�ң���ʯ�ҵ�����������3.06g������ȼ�ղ���ͨ��Ũ���ᣬŨ���������������1.08g��ȡ4.6gA�������Ľ����Ʒ�Ӧ�����ɵ������ڱ�״���µ����Ϊ1.68L��A���봿�Ӧ��ͨ������ȷ��A�ķ���ʽ�ͽṹ��ʽ����д����Ҫ������˵����������̣�
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
�������л���A�����ĺ����������ͬ��ͬѹ�£�A�������Ҵ�����������ܶ���2����A����Է�������Ϊ92��1.38gA��ȫȼ�պ�����ȼ�յIJ���ͨ����ʯ�ң���ʯ�ҵ�����������3.06gΪ������̼��ˮ������������ȼ�ղ���ͨ��Ũ���ᣬŨ���������������1.08gΪˮ���������ʶ�����̼����Ϊ3.06g-1.08g=1.98g������ԭ���غ�����л���A��C��Hԭ����Ŀ���ٽ��A����Է����������������Oԭ����Ŀ���ݴ�ȷ���л���A�ķ���ʽ��
ȡ4.6gA�����ʵ���Ϊ0.05mol���������Ľ����Ʒ�Ӧ�����ɵ������ڱ�״���µ����Ϊ1.68L�������������ʵ���Ϊ
=0.075mol��A���봿�Ӧ����Aû��-COOH�����л��ﺬ��-OH���ǻ���Ŀ=
=3���ݴ˽��
ȡ4.6gA�����ʵ���Ϊ0.05mol���������Ľ����Ʒ�Ӧ�����ɵ������ڱ�״���µ����Ϊ1.68L�������������ʵ���Ϊ
| 1.68L |
| 22.4L/mol |
| 0.075mol��2 |
| 0.05mol |
���
�⣺�л���A�����ĺ����������ͬ��ͬѹ�£�A�������Ҵ�����������ܶ���2����A����Է�������Ϊ92��
1.38gA�����ʵ���=
=0.015mol����ȫȼ�պ�����ȼ�յIJ���ͨ����ʯ�ң���ʯ�ҵ�����������3.06gΪ������̼��ˮ������������ȼ�ղ���ͨ��Ũ���ᣬŨ���������������1.08gΪˮ��������ˮ�����ʵ���=
=0.06mol��������̼����Ϊ3.06g-1.08g=1.98g�������ʵ���=
=0.045mol��
���л���A��Cԭ����Ŀ=
=3��Hԭ����Ŀ=
=8����A������Oԭ����Ŀ=
=3���л���A�ķ���ʽΪC3H8O3��
ȡ4.6gA�����ʵ���Ϊ
=0.05mol���������Ľ����Ʒ�Ӧ�����ɵ������ڱ�״���µ����Ϊ1.68L�������������ʵ���Ϊ
=0.075mol��A���봿�Ӧ�����л��ﺬ��-OH���ǻ���Ŀ=
=3����A�Ľṹ��ʽΪ��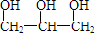 ��
��
���л���A�ķ���ʽΪC3H8O3���ṹ��ʽΪ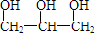 ��
��
1.38gA�����ʵ���=
| 1.38 |
| 92g/mol |
| 1.08g |
| 18g/mol |
| 1.98g |
| 44g/mol |
���л���A��Cԭ����Ŀ=
| 0.045mol |
| 0.015mol |
| 0.06mol��2 |
| 0.015mol |
| 92-12��3-8 |
| 16 |
ȡ4.6gA�����ʵ���Ϊ
| 4.6g |
| 92g/mol |
| 1.68L |
| 22.4L/mol |
| 0.075mol��2 |
| 0.05mol |
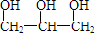 ��
�����л���A�ķ���ʽΪC3H8O3���ṹ��ʽΪ
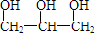 ��
��
���������⿼���л������ʽ��ṹȷ�������ڼ������ƶϣ�����ԭ���غ�ȷ���л������ʽ�ǹؼ���ע�����ճ��������ŵ����ʣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�������ʢٹ��˺����Ȼˮ���ڱ���ǵ�ʯ��ˮ����ʯ����Һ����ţ�̡������ᡡ��ƣ�����������Һ���ǣ�������
| A���٢� | B���ڢ� |
| C���ۢݢ� | D���٢ۢݢ� |
������������ȷ���ǣ�������
| A��ͬϵ�ﲻ������ͬ���칹�� |
| B��CH3Cl��CHCl3��Ϊͬϵ�� |
| C��ͬ���칹�岻�ܻ���Ϊͬϵ�� |
| D��C4H10�������ĸ�̼ԭ����һ��ֱ���� |
���б仯�����У���Ҫ���뻹ԭ�����ǣ�������
| A��HCl��H2 |
| B��FeCl2��FeCl3 |
| C��Na2SO3��SO2 |
| D��Fe��Fe3O4 |