��Ŀ����
��֪������Ϣ����Ũ�����д���ƽ�⣺H2SO4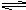 H++HSO4- HSO4-
H++HSO4- HSO4-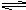 H++SO42-��PbSO4������ˮ��HNO3����������Ũ����ʹ������Һ����ش������й����⣺
H++SO42-��PbSO4������ˮ��HNO3����������Ũ����ʹ������Һ����ش������й����⣺
��1�����Թ��м�������PbSO4������������ˮ��������Һ����������Һ�м�����������粒��壬��õ�������Һ���÷�Ӧ������ԭ������� ����д���÷�Ӧ�����ӷ���ʽ�� ��
��2�����ó�����Һ��ͨ��H2S���壬�к�ɫ����(PbS)���ɣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������ʵ���Ʋ⣬PbSO4��PbS���ܽ�� ��
��4��������PbSO4��ĩͶ��ʢŨ������Թܣ���Ҳ�ó�����Һ������ƽ���ƶ�ԭ������PbSO4������Ũ�����ԭ�� ��
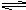 H++HSO4- HSO4-
H++HSO4- HSO4-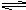 H++SO42-��PbSO4������ˮ��HNO3����������Ũ����ʹ������Һ����ش������й����⣺
H++SO42-��PbSO4������ˮ��HNO3����������Ũ����ʹ������Һ����ش������й����⣺��1�����Թ��м�������PbSO4������������ˮ��������Һ����������Һ�м�����������粒��壬��õ�������Һ���÷�Ӧ������ԭ������� ����д���÷�Ӧ�����ӷ���ʽ�� ��
��2�����ó�����Һ��ͨ��H2S���壬�к�ɫ����(PbS)���ɣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������ʵ���Ʋ⣬PbSO4��PbS���ܽ�� ��
��4��������PbSO4��ĩͶ��ʢŨ������Թܣ���Ҳ�ó�����Һ������ƽ���ƶ�ԭ������PbSO4������Ũ�����ԭ�� ��
��1��(CH3COO)2Pb���ѵ�������ʣ�PbSO4+2 CH3COO -
 (CH3COO)2Pb+SO42-
(CH3COO)2Pb+SO42-��2��(CH3COO)2Pb��H2S=PbS����2CH3COOH
��3��PbSO4
��4����PbSO4Ͷ��Ũ�����У�Ũ������H+���SO42-����HSO4-������ƽ��PbSO4��s��
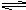 Pb2+��aq��+ SO42-��aq�������ƶ�����ˣ�PbSO4������Ũ���ᡣ
Pb2+��aq��+ SO42-��aq�������ƶ�����ˣ�PbSO4������Ũ���ᡣ������Ҫ����ѧ����������ͽ�������������PbSO4������Һ�м������ó�����Һ�����ݻ��ϼ۹����߲�����������ԭ��Ӧ����ط������ֽⷴӦ����ֻ���������������ѵ�������ʣ�ֻ���Ǵ���Ǧ�ˡ�ͨ���������ܵõ���Ǧ���������൱������������ӡ������Ӻ�Ǧ����ͬʱ����ʱ����Ǧ�ȳ���������Ǧ���ܽ�ȸ�С��

��ϰ��ϵ�д�
�����Ŀ
 B(OCH3)3 +3H2O�У�H3BO 3��ת���ʣ�
B(OCH3)3 +3H2O�У�H3BO 3��ת���ʣ�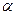 ���ڲ�ͬ�¶����淴Ӧʱ�䣨t���ı仯��ͼ12���ɴ�ͼ�ɵó���
���ڲ�ͬ�¶����淴Ӧʱ�䣨t���ı仯��ͼ12���ɴ�ͼ�ɵó���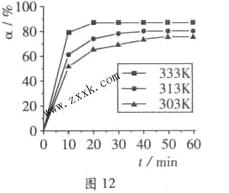
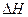 _____0(�<������=����>��).
_____0(�<������=����>��). H)4]-( aq)+H+��aq����֪0.70 mol��L-1 H3BO 3��Һ�У�������Ӧ��298K�ﵽƽ��ʱ��cƽ����H+��="2." 0 �� 10-5mol��L-1��cƽ����H3BO 3����c��ʼ��H3BO 3����ˮ�ĵ���ɺ��Բ��ƣ�����¶��¸÷�Ӧ��ƽ�ⳣ��K��H2O��ƽ��Ũ�Ȳ�����K�ı���ʽ�У�������������λ��Ч���֣�
H)4]-( aq)+H+��aq����֪0.70 mol��L-1 H3BO 3��Һ�У�������Ӧ��298K�ﵽƽ��ʱ��cƽ����H+��="2." 0 �� 10-5mol��L-1��cƽ����H3BO 3����c��ʼ��H3BO 3����ˮ�ĵ���ɺ��Բ��ƣ�����¶��¸÷�Ӧ��ƽ�ⳣ��K��H2O��ƽ��Ũ�Ȳ�����K�ı���ʽ�У�������������λ��Ч���֣�
 FeO(s)+CO(g) Fe(s)+CO2(g) ��H=-1kJ/mol��
FeO(s)+CO(g) Fe(s)+CO2(g) ��H=-1kJ/mol��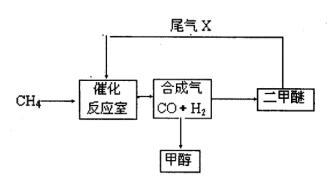
 ��2��β��ѭ�����漰�����·�Ӧ��CH4��g����H2O��g�� CO��g����3H2��g��,��д��ij�¶��¸÷�Ӧ��ƽ�ⳣ������ʽ ��
��2��β��ѭ�����漰�����·�Ӧ��CH4��g����H2O��g�� CO��g����3H2��g��,��д��ij�¶��¸÷�Ӧ��ƽ�ⳣ������ʽ �� ��Ӧ�� CO��g����2H2��g�� CH3OH��g��
��Ӧ�� CO��g����2H2��g�� CH3OH��g�� 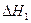
 ��Ӧ��CO2��g����3H2��g�� CH3��g����H2O��g��
��Ӧ��CO2��g����3H2��g�� CH3��g����H2O��g��
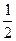 O2��g��
O2��g��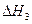 =��192.9kJ��mol��1
=��192.9kJ��mol��1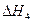 =��241.8kJ��mol��1
=��241.8kJ��mol��1 CO��g��+ H2O��g�� ��H =" Q" kJ��mol��1
CO��g��+ H2O��g�� ��H =" Q" kJ��mol��1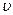 ����H2����
����H2����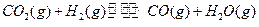 ���ﵽ�����ʱ��90%�����������������ת��Ϊˮ����֪�ڸ�״̬�´��ڹ�ϵ
���ﵽ�����ʱ��90%�����������������ת��Ϊˮ����֪�ڸ�״̬�´��ڹ�ϵ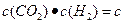
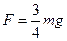 �����������ж�����̼�������������Ϊ�� ��
�����������ж�����̼�������������Ϊ�� �� 3Y��g��+Z��g��,�ﵽƽ��ʱ����30%��X�����ֽ⣬��ƽ��ʱ��������ܵ����ʵ����ǣ� ��
3Y��g��+Z��g��,�ﵽƽ��ʱ����30%��X�����ֽ⣬��ƽ��ʱ��������ܵ����ʵ����ǣ� �� Ca2++2OH-����������Һ�м���������ʯ�Һ����¶ȱ��ֲ��䣬�����ж���ȷ���ǣ� ��
Ca2++2OH-����������Һ�м���������ʯ�Һ����¶ȱ��ֲ��䣬�����ж���ȷ���ǣ� ��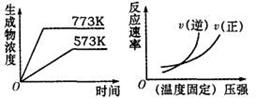
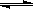 2NH3(g) ����H=��Q1kJ��mol��1(Q1>0)
2NH3(g) ����H=��Q1kJ��mol��1(Q1>0)