��Ŀ����
6�����з�Ӧ�У�����ȡ����Ӧ���ǣ���������CH3CH=CH2+Br��CH3CHBrCH2Br
��CH3CH2OH$��_{��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O
��CH3COOH+CH3CH2OH $��_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O
��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+H2O��
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
���� �л����е�ԭ�ӻ�ԭ���ű�������ԭ�ӻ�ԭ���������������µĻ�����ķ�Ӧ��ȡ����Ӧ�����ݶ��������
��� �⣺��CH3CH�TCH2�е�̼̼˫�����ѣ�ÿ��̼ԭ���Ϸֱ���һ��Brԭ�ӣ�����CH3CHBrCH2Br�����ڼӳɷ�Ӧ���ʢٴ���
��CH3CH2OH������ȥ��Ӧ��ʧȥ1����ˮ���ʢڴ���
��CH3COOH�ϵ��ǻ���CH3CH2O-ȡ������CH3COOCH2CH3������ȡ����Ӧ���ʢ���ȷ��
��C6H6�б����ϵ���ԭ�ӱ���ԭ��ȡ�������屽������ȡ����Ӧ���ʢ���ȷ��
��ѡB��
���� ���⿼��ѧ���л���Ӧ�����е�ȡ����Ӧ���ѶȲ����Ը�����ѧ֪ʶ���У�

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ
13��������ʵ�������л�������ڻ��ż������ý��͵��ǣ�������
| A�� | ���ӣ�C6H5OH���ܸ�NaOH��Һ��Ӧ���Ҵ����� | |
| B�� | ��ϩ�ܷ����ӳɷ�Ӧ�����鲻�� | |
| C�� | �ױ���ʹKMnO4������Һ��ɫ�����鲻�� | |
| D�� | ����50�桫60��ʱ����������Ӧ���ױ���30��ʱ���� |
14����1L������100L�����г��ȼ�գ���Ӧ�������徭Ũ��������ʣ����������Ϊ�������������ͬ�����²ⶨ����������
| A�� | 93.5L | B�� | 95L | C�� | 97.5L | D�� | 98.5L |
11������ʽΪC7H8���ҷ����к��б���������һ�ȴ����У�������
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 7�� |
1���������ʵ���������������Ŷ���ȷ���ǣ�������
| A�� | 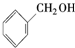 ����-OH ����-OH | B�� | 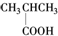 ����-COOH ����-COOH | ||
| C�� | 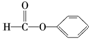 ȩ��-CHO ȩ��-CHO | D�� | CH3-O-CH3������-CHO |
18��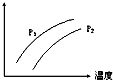 ��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������
��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������
 ��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������
��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������| A�� | �������L�İٷֺ��� | B�� | ���������ܶ� | ||
| C�� | L��ת���� | D�� | ��������ƽ�������� |
15����������������ʵ�з���Ͷ�ţ����з����ϡ�������Ʒ�����ڣ�������
| A�� | ���� | B�� | ��� | C�� | ���� | D�� | ���� |
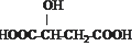
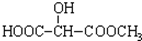 B��
B��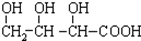 C��H3COOC-COOCH3
C��H3COOC-COOCH3 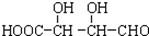 E��
E��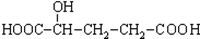
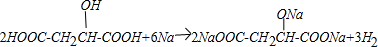 ��
��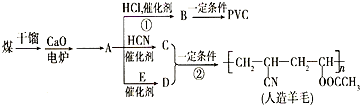 ������������ʯ�ͼ۸����ǣ���úΪԭ���Ʊ�һЩ������Ʒ��ǰ���ֱ����ã���ͼ����úΪԭ��������PVC����������ë�ĺϳ�·�ߣ�
������������ʯ�ͼ۸����ǣ���úΪԭ���Ʊ�һЩ������Ʒ��ǰ���ֱ����ã���ͼ����úΪԭ��������PVC����������ë�ĺϳ�·�ߣ�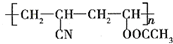 ��
��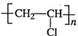 ��
��