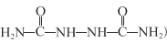ΧβΡΩΡΎ»ί
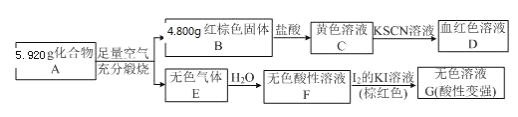
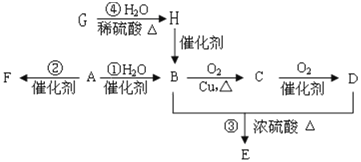
ΓΨΧβΡΩΓΩA « ·”ΆΝ―ΫβΤχΒΡ÷ς“Σ≥…Ζ÷Θ§AΒΡ≤ζΝΩΆ®≥Θ”Οά¥ΚβΝΩ“ΜΗωΙζΦ“ΒΡ ·”ΆΜ·ΙΛΖΔ’ΙΥ°ΤΫΘΜF «“Μ÷÷ΗΏΨέΈοΘ§≥ΘΉω ≥ΤΖΑϋΉΑ¥ϋΒΡ≤ΡΝœΘΜG”ωΒΫΒβΥ°Ρή±δ≥…άΕ…ΪΘΜE «ΨΏ”–Υ°ΙϊœψΈΕΒΡΜ·ΚœΈοΓΘ‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§”–ΜζΈοAΓΔBΓΔCΓΔDΓΔEΓΔFΓΔGΓΔHΦδ¥φ‘Ύœ¬Ν–±δΜ·ΙΊœΒΓΘ
Θ®1Θ©AΓΔDΖ÷Ή”÷–ΒΡΙΌΡήΆ≈Οϊ≥ΤΖ÷±π «________ΓΔ_________ΓΘ
Θ®2Θ©H»ή“Κ÷–Φ”»κ–¬÷ΤΒΡCu(OH)2≤ΔΦ”»»Θ§Ω…Ιέ≤λΒΫΒΡœ÷œσ «_________Θ§“Ϋ―ß…œάϊ”Ο¥Υ‘≠άμΩ…”Οά¥Φλ≤β__________ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΎΓΔΔέΒΡΜ·―ßΖΫ≥Χ ΫΘ§≤Δ÷Η≥ωΖ¥”Πάύ–ΆΘΚ
ΔΎ_________________Θ§__________ΘΜ
Δέ_________________Θ§__________ΓΘ
ΓΨ¥πΑΗΓΩΧΦΧΦΥΪΦϋ τ»Μυ ”–Ή©Κλ…Ϊ≥ΝΒμ…ζ≥… Χ«Ρρ≤Γ nCH2=CH2 ![]()
![]() Φ”ΨέΖ¥”Π CH3COOH+CH3CH2OH
Φ”ΨέΖ¥”Π CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O θΞΜ·Ζ¥”ΠΘ®Μρ’Ώ»Γ¥ζΖ¥”ΠΘ©
CH3COOCH2CH3+H2O θΞΜ·Ζ¥”ΠΘ®Μρ’Ώ»Γ¥ζΖ¥”ΠΘ©
ΓΨΫβΈωΓΩ
A « ·”ΆΝ―ΫβΤχΒΡ÷ς“Σ≥…ΖίΘ§AΒΡ≤ζΝΩΆ®≥Θ”Οά¥ΚβΝΩ“ΜΗωΙζΦ“ΒΡ ·”ΆΜ·ΙΛΖΔ’ΙΥ°ΤΫΘ§‘ρAΈΣCH2=CH2Θ§F «“Μ÷÷ΗΏΨέΈοΘ§AΖΔ…ζΦ”ΨέΖ¥”Π…ζ≥…ΒΡFΈΣ![]() Θ§A”κΥ°ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…BΘ§BΈΣCH3CH2OHΘ§““¥Φ‘ΎCuΉς¥ΏΜ·ΦΝΧθΦΰœ¬ΖΔ…ζ―θΜ·Ζ¥”Π…ζ≥…CΘ§CΈΣCH3CHOΘ§CΫχ“Μ≤Ϋ―θΜ·…ζ≥…DΘ§DΈΣCH3COOHΘ§E «ΨΏ”–Υ°ΙϊœψΈΕΒΡΜ·ΚœΈοΘ§CH3CH2OHΚΆCH3COOH‘Ύ≈®ΝρΥαΉς”Οœ¬Ζ¥”Π…ζ≥…EΘ§EΈΣCH3COOCH2CH3Θ§G”ωΒΫΒβΥ°Ρή±δ≥…άΕ…ΪΘ§GΈΣΒμΖέΘ§Υ°ΫβΒΟΒΫHΘ§HΈΣΤœΧ―Χ«Θ§ΤœΧ―Χ«Ω…“‘ΉΣΜ·ΒΟΒΫ““¥ΦΘ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
Θ§A”κΥ°ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…BΘ§BΈΣCH3CH2OHΘ§““¥Φ‘ΎCuΉς¥ΏΜ·ΦΝΧθΦΰœ¬ΖΔ…ζ―θΜ·Ζ¥”Π…ζ≥…CΘ§CΈΣCH3CHOΘ§CΫχ“Μ≤Ϋ―θΜ·…ζ≥…DΘ§DΈΣCH3COOHΘ§E «ΨΏ”–Υ°ΙϊœψΈΕΒΡΜ·ΚœΈοΘ§CH3CH2OHΚΆCH3COOH‘Ύ≈®ΝρΥαΉς”Οœ¬Ζ¥”Π…ζ≥…EΘ§EΈΣCH3COOCH2CH3Θ§G”ωΒΫΒβΥ°Ρή±δ≥…άΕ…ΪΘ§GΈΣΒμΖέΘ§Υ°ΫβΒΟΒΫHΘ§HΈΣΤœΧ―Χ«Θ§ΤœΧ―Χ«Ω…“‘ΉΣΜ·ΒΟΒΫ““¥ΦΘ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
(1)AΒΡΫαΙΙΦρ ΫΈΣCH2=CH2Θ§Κ§”–ΧΦΧΦΥΪΦϋΘ§DΒΡΫαΙΙΦρ ΫΈΣCH3COOHΘ§Κ§”–τ»ΜυΘ§Ι ¥πΑΗΈΣΘΚΧΦΧΦΥΪΦϋΘΜτ»ΜυΘΜ
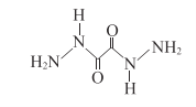
(2)HΈΣΤœΧ―Χ«(C6H12O6)Θ§œρHΒΡΥ°»ή“Κ÷–Φ”»κ–¬÷ΤΒΡCu(OH)2≤ΔΦ”»» ±≤ζ…ζΒΡ Β―ιœ÷œσ «”–Ή©Κλ…Ϊ≥ΝΒμ≤ζ…ζΘ§“Ϋ―ß…œάϊ”Ο¥Υ‘≠άμΩ…”Οά¥Φλ≤βΧ«Ρρ≤ΓΘ§Ι ¥πΑΗΈΣΘΚ”–Ή©Κλ…Ϊ≥ΝΒμ≤ζ…ζΘΜΧ«Ρρ≤ΓΘΜ
(3)Ζ¥”ΠΔΎΒΡΖΫ≥Χ ΫΈΣΘΚnCH2=CH2 ![]()
![]() Θ§ τ”ΎΦ”ΨέΖ¥”ΠΘΜΖ¥”ΠΔέΒΡΖΫ≥Χ ΫΈΣΘΚCH3COOH+HOC2H5
Θ§ τ”ΎΦ”ΨέΖ¥”ΠΘΜΖ¥”ΠΔέΒΡΖΫ≥Χ ΫΈΣΘΚCH3COOH+HOC2H5 ![]() CH3COOC2H5+H2OΘ§ τ”Ύ»Γ¥ζΖ¥”ΠΜρθΞΜ·Ζ¥”ΠΘΜΙ ¥πΑΗΈΣΘΚnCH2=CH2
CH3COOC2H5+H2OΘ§ τ”Ύ»Γ¥ζΖ¥”ΠΜρθΞΜ·Ζ¥”ΠΘΜΙ ¥πΑΗΈΣΘΚnCH2=CH2 ![]()
![]() ΘΜΦ”ΨέΖ¥”ΠΘΜCH3COOH+HOC2H5
ΘΜΦ”ΨέΖ¥”ΠΘΜCH3COOH+HOC2H5 ![]() CH3COOC2H5+H2OΘΜ»Γ¥ζΖ¥”ΠΜρθΞΜ·Ζ¥”ΠΓΘ
CH3COOC2H5+H2OΘΜ»Γ¥ζΖ¥”ΠΜρθΞΜ·Ζ¥”ΠΓΘ