��Ŀ����
����Ŀ��ʯ���ѽ�����Ҫ���б�ϩ��1,3-����ϩ�Ȳ���������������Ϊԭ�Ͽɺϳ�CR��ҽҩ�м���G,���ϳ�·�����£�

��֪����B��C��D ���ܷ���������Ӧ��
��
��1��A��˳ʽ�칹��Ľṹ��ʽΪ___________________��
��2��C�к��������ŵ�������____________����Ӧ�ٵķ�Ӧ����Ϊ____________________��
��3��д��E�� ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
��4��д��ͬʱ��������������ҽҩ�м���G��ͬ���칹��Ľṹ��ʽ�� __________________��
����D ��Ϊͬϵ� �ں˴Ź�������������塣
��5���ü�Ҫ���Ա�������B�����������ŵ�ʵ�鷽����_______________________��
��6����AΪ��ʼԭ�Ϻϳ�CR����·Ϊ______________________�������Լ���ѡ����
���𰸡� 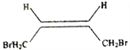 �ǻ���ȩ�� ȡ����Ӧ HOOCCH2COOH+2C2H5OH
�ǻ���ȩ�� ȡ����Ӧ HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫��
C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫�� 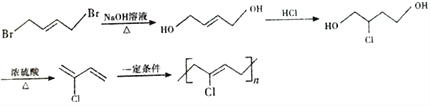
����������������ͼ��1,3-����ϩ���巢��1,4�ӳɣ�����A��AΪ1,4-����-2-��ϩ(![]() )���������ӳɺ�����1,4-���嶡��(
)���������ӳɺ�����1,4-���嶡��(![]() )����ϩ����������B��BΪCH2=CHCHO��C����Է���������B��18��˵��B��ˮ�ӳ�����C��CΪ
)����ϩ����������B��BΪCH2=CHCHO��C����Է���������B��18��˵��B��ˮ�ӳ�����C��CΪ![]() ������������D(����ȩ)����������Һ��Ӧ����E(������)�����Ҵ�������Ӧ����F(
������������D(����ȩ)����������Һ��Ӧ����E(������)�����Ҵ�������Ӧ����F(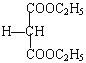 )��������Ϣ��F(
)��������Ϣ��F( )��
)��![]() ��Ӧ����
��Ӧ����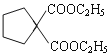 ����һ����Ӧ����G(
����һ����Ӧ����G(![]() )��
)��
(1)AΪ1,4-����-2-��ϩ(![]() )����˳ʽ�칹��Ľṹ��ʽΪ
)����˳ʽ�칹��Ľṹ��ʽΪ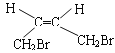 ����ȷ����
����ȷ���� ��
��
(2) CΪ![]() �����еĹ�������̼̼�ǻ���ȩ����ͨ�������Ϣ�ڿ�֪���ù��̷�����ȡ����Ӧ����ȷ����̼̼˫����ȩ���� ȡ����Ӧ��
�����еĹ�������̼̼�ǻ���ȩ����ͨ�������Ϣ�ڿ�֪���ù��̷�����ȡ����Ӧ����ȷ����̼̼˫����ȩ���� ȡ����Ӧ��
��3���л���EΪHOOCCH2COOH����C2H5OH��Ũ������ȵ������·���������Ӧ����ѧ����ʽ��HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O ����ȷ����HOOCCH2COOH+2C2H5OH
C2H5OOCCH2COOC2H5+2H2O ����ȷ����HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O��
C2H5OOCCH2COOC2H5+2H2O��
��4���л���G�ķ���ʽΪC6H10O2, ����D ��Ϊͬϵ��л���DΪ��Ԫȩ�ࣻ�ں˴Ź�������������壬����һ���ĶԳ��ԣ������������л�����ܵĽṹ��2�֣�OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO����ȷ�𰸣� OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO��
��5���л���B CH2=CHCHO������ȩ����̼̼˫��������ȩ���Ļ�ԭ�Խ�ǿ���ȼ���ȩ����Ȼ���ڼ���̼̼˫�����������������ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫�� ����ȷ����ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һɫ��֤������̼̼˫�� ��
��6��AΪ![]() ���ڼ��Ի����·���ȡ����Ӧ���ɶ�Ԫϩ��
���ڼ��Ի����·���ȡ����Ӧ���ɶ�Ԫϩ��![]() ��Ȼ�������Ȼ��ⷢ���ӳ�����
��Ȼ�������Ȼ��ⷢ���ӳ�����![]() �����л�����Ũ���������·�����ȥ��Ӧ����
�����л�����Ũ���������·�����ȥ��Ӧ����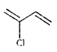 �����л�����Ӿ۷�Ӧ���ɸ߷��ӣ������������£�
�����л�����Ӿ۷�Ӧ���ɸ߷��ӣ������������£�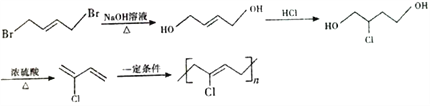 ����ȷ�𰸣�
����ȷ�𰸣� ��
��

����Ŀ������SO2��CO��NOx��Ⱦ�ǻ�ѧ�������о�����Ҫ���⡣
��.���᳧�����ŷź�SO2��β����Ի����������Σ����
��1����ҵ�Ͽ����÷ϼ�Һ����Ҫ�ɷ�ΪNa2CO3���������᳧β���е�SO2���õ�Na2SO3��Һ���÷�Ӧ�����ӷ���ʽΪ__________________________________��
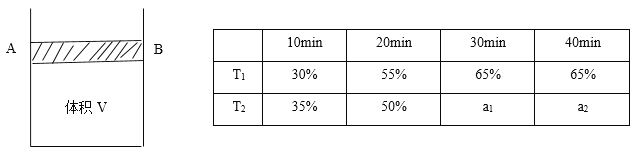
��.�������������Ϊ��Ӧ��2CO(g)+O2(g)![]() 2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��

��2��a��b��c��d �ĵ��У��ﵽƽ��״̬����__________________________________��
��3����֪c��ʱ������O2Ũ��Ϊ0.02 mol/L����50��ʱ���ڦ��������������COת����Ӧ��ƽ�ⳣ��K=____________���ú�x�Ĵ���ʽ��ʾ����
��4�����й���ͼ��˵����ȷ����_____________��
A.COת����Ӧ��ƽ�ⳣ��K(a)
B.�ھ�δ�ﵽƽ��״̬ʱ��ͬ���¦��������������COת�����ʱȦ���Ҫ��
C.b��ʱCO��O2����֮�䷢����Ч��ײ�ļ���������ʵ����������
D.e��ת���ʳ���ͻ���ԭ��������¶����ߺ����ʧȥ����
��.ij���ܴ������Դ��������ͳ�β���е�̼��(C)��NOx����ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô�����������в��CO2��N2��N2O����NO��������ݽ����ͼ����ʾ��
ģ��β�� | ����(10mol) | ̼�� | ||
NO | O2 | He | ||
���ʵ���(mol) | 0.025 | 0.5 | 9.475 | n |
��5��375��ʱ������ų��������к�0.45 molO2��0.0525 mol CO2����Y�Ļ�ѧʽΪ______________��

��6��ʵ������в���NOģ��NOx����������NO2��ԭ����____________________________��