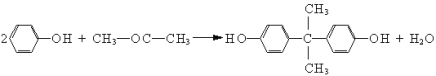
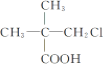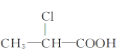��Ŀ����
����Ŀ��ij�Ȼ�����Ʒ�к�������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

������������̣��ش��������⣺
��1������I������һ�����ʵ���Ũ�ȵ���Һ�����õ��IJ����������ձ����������⣬��������_________��__________�����������ƣ���
��2�����в�������ʹ������ҺŨ��ƫС����___________����д��ţ���
��δϴ���ձ��Ͳ�����
�ڶ���ʱ��������ƿ�Ŀ̶���
������Һǰ����ƿ������������ˮ
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶�������
��3����д����ͼ�еμ���ˮ����������Ӧ�����ӷ���ʽ____________________��
��4����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ�����____________��
��5����Ʒ����Ԫ�ص�����������_____________��
���𰸡�250mL����ƿ ��ͷ�ι� �٢� 2Fe 2+ ��Cl2 �� 2Fe 3+ �� 2Cl- ȡ�������ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ� 39.2%
��������
��1������ʵ��Ĺ淶��������ѡȡ����IJ���������
��2���������ʵ���Ũ�ȹ�ʽc=![]() �����������淶��Ũ������ɵ�Ӱ��Ч����
�����������淶��Ũ������ɵ�Ӱ��Ч����
��3����ˮ�������������������ӣ�
��4��������Һ�п���ʣ��IJ������ӷ�������
��5��������Ԫ���غ㣬������ʵ�����صļ��������
��1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�250.0mL��Һ����Ҫ250mL����ƿ�����ⶨ��ʱ����Ҫ��ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����δϴ���ձ��Ͳ����������Ƶ��������ʵ���ƫС������c=![]() ��֪��������ҺŨ��ƫС��������ȷ��
��֪��������ҺŨ��ƫС��������ȷ��
�ڶ���ʱ��������ƿ�Ŀ̶��ߣ���Һ���ƫС������c=![]() ��֪��������ҺŨ��ƫ�������
��֪��������ҺŨ��ƫ�������
������Һǰ����ƿ������������ˮ������Һ��Ũ����Ӱ�죬�������
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶������У��൱��ϡ����Һ��ʹ������ҺŨ��ƫС��������ȷ��
�ʴ�Ϊ���٢ܣ�
��3���μ���ˮ������������������ת��Ϊ�����ӣ������ӷ���ʽΪ��2Fe2+��Cl2��2Fe 3+��2Cl-��
��4����Һ�д����Ȼ�泥����������ữ����������Һ�������һ��ϴ��Һ���Ƿ���������ӣ��ʴ�Ϊ��ȡ�������ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��5������Ԫ�������غ㣬������ɫ�����е���������Ʒ��������Fe2O3������Ϊ2.8g�����ڲμӷ�Ӧ����Һֻȡ������Һ��1/10�������Ԫ�ص�����Ϊ10��2.8g��![]() =19.6g����Ʒ����Ԫ�ص�����������
=19.6g����Ʒ����Ԫ�ص�����������![]() =39.2%���ʴ�Ϊ��39.2%��
=39.2%���ʴ�Ϊ��39.2%��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�