��Ŀ����
��13�֣��Ը����ϱ���գ�
��1����д��Ԫ��Q�Ļ�̬ԭ�ӵ����Ų�ʽ ��Ԫ��N�����ڱ��е�λ�� ���� �塣
��2����̬Dԭ�Ӻ����� ��δ�ɶԵ��ӣ�D2�����зֱ��� ���� ���м���
��3��������DA5�мȺ����Ӽ����ֺ����ۼ�����λ������д�����ĵ���ʽ ��
��4�������е�Ԫ�����γ�XY2�͵Ĺ��۷�����CE2��CL2��BK2��LE2���֣�����ӵĿռ乹������һ�����������ֲ�ͬ������ ���ѧʽ�������γ�XY3�͵Ĺ��۷���Ҳ�����֣����Ƿֱ���DA3��DF3��DK3��LE3������ӵĿռ乹����Ҳ��һ�����������ֲ�ͬ������ ���ѧʽ��,������_ ��������ԡ��Ǽ��ԡ�����ͬ�����ɵ� ���ӡ�
��5����������8��Ԫ�ذ������۵�ߵ͵�˳������ͼ��ʾ��������š�8����������Ԫ���� ����Ԫ�ط��ţ���������̬�⻯���ȶ�����ǿ��Ԫ���� ������ͼ�е���ţ���

��6����ѧ��֤ʵ��IK3���ڹ��ۻ��������ʽΪI2K6����ṹʽΪ���������е�һ�֣�����Ϊ��ȷ�Ľṹʽ��____________��

��7����Q����������Һ�м���������DA3��ˮ��Һ�����У���д��������Ӧ�����ӷ���ʽ ��������������ӵĽṹʽΪ ��
| A | | | ||||||||||||||||
| | B | | | C | D | E | F | | ||||||||||
| G | H | I | J | | L | K | M | |||||||||||
| | | | | | | | N | | | Q | | | | | | | | |
��2����̬Dԭ�Ӻ����� ��δ�ɶԵ��ӣ�D2�����зֱ��� ���� ���м���
��3��������DA5�мȺ����Ӽ����ֺ����ۼ�����λ������д�����ĵ���ʽ ��
��4�������е�Ԫ�����γ�XY2�͵Ĺ��۷�����CE2��CL2��BK2��LE2���֣�����ӵĿռ乹������һ�����������ֲ�ͬ������ ���ѧʽ�������γ�XY3�͵Ĺ��۷���Ҳ�����֣����Ƿֱ���DA3��DF3��DK3��LE3������ӵĿռ乹����Ҳ��һ�����������ֲ�ͬ������ ���ѧʽ��,������_ ��������ԡ��Ǽ��ԡ�����ͬ�����ɵ� ���ӡ�
��5����������8��Ԫ�ذ������۵�ߵ͵�˳������ͼ��ʾ��������š�8����������Ԫ���� ����Ԫ�ط��ţ���������̬�⻯���ȶ�����ǿ��Ԫ���� ������ͼ�е���ţ���

��6����ѧ��֤ʵ��IK3���ڹ��ۻ��������ʽΪI2K6����ṹʽΪ���������е�һ�֣�����Ϊ��ȷ�Ľṹʽ��____________��

��7����Q����������Һ�м���������DA3��ˮ��Һ�����У���д��������Ӧ�����ӷ���ʽ ��������������ӵĽṹʽΪ ��
��1����2�֣�1s22s22p63s23p63d104s1�� ��2�֣���4���� VIII�塣
��2����3�֣� 3�� 1 �� 2����3����2�֣�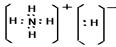 ��
��
��4����4�֣� SO2�� SO3 �� ���ԣ� �Ǽ���
��5����2�֣� Si �� 2 ����6����2�֣� �� ��
��7����6�֣�Cu2++2NH3?H2O= Cu(OH)2��+2NH4+
Cu(OH)2 +4NH3 =��Cu(NH3)4��2+ + 2OH-��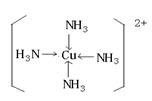
��2����3�֣� 3�� 1 �� 2����3����2�֣�
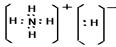 ��
����4����4�֣� SO2�� SO3 �� ���ԣ� �Ǽ���
��5����2�֣� Si �� 2 ����6����2�֣� �� ��
��7����6�֣�Cu2++2NH3?H2O= Cu(OH)2��+2NH4+
Cu(OH)2 +4NH3 =��Cu(NH3)4��2+ + 2OH-��

��1��Q��ͭ�����ݹ���ԭ����֪�������Ų�ʽΪ1s22s22p63s23p63d104s1��N����Ԫ�أ�λ�ڵ�4����VIII�塣
��2��D�ǵ�Ԫ�أ��������3��δ�ɶԵ��ӡ����������������ɵģ�����������2���м���1���Ҽ����ɡ�
��3��������DA5���⻯泥��������ӻ��������ʽΪ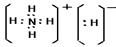 ��
��
��4��CE2��CL2��BK2��LE2�������ʷֱ���CO2��CS2��BeCl2��SO2������ǰ������ֱ���ͻ����SO2��V�νṹ��DA3��DF3��DK3��LE3�������ʷֱ���NH3��NF3��NF3��SO3������SO3��ƽ�������Σ����ɼ��Լ��γɵķǼ��Է��ӣ�����������Ρ�
��5������ԭ�Ӿ��壬�۵���ߡ���Ԫ�صķǽ�������ǿ���Ȼ������ȶ��������ȵķе�֮��Ar�ĸߣ�������2��
��6����Ԫ������㺬�й¶Ե��ӣ��ܺ���Ԫ���γ���λ����������ȷ�Ĵ��Ǣۡ�
��7������ͭ�Ͱ�ˮ�������ֽⷴӦ������������ͭ����������ˮ����ʱ�����ڰ����ܺ�ͭ�����γ���λ�������Գ������ܽ⣬����������ӦʽΪCu2++2NH3?H2O= Cu(OH)2��+2NH4+��Cu(OH)2 +4NH3 =��Cu(NH3)4��2+ + 2OH-��
��2��D�ǵ�Ԫ�أ��������3��δ�ɶԵ��ӡ����������������ɵģ�����������2���м���1���Ҽ����ɡ�
��3��������DA5���⻯泥��������ӻ��������ʽΪ
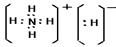 ��
����4��CE2��CL2��BK2��LE2�������ʷֱ���CO2��CS2��BeCl2��SO2������ǰ������ֱ���ͻ����SO2��V�νṹ��DA3��DF3��DK3��LE3�������ʷֱ���NH3��NF3��NF3��SO3������SO3��ƽ�������Σ����ɼ��Լ��γɵķǼ��Է��ӣ�����������Ρ�
��5������ԭ�Ӿ��壬�۵���ߡ���Ԫ�صķǽ�������ǿ���Ȼ������ȶ��������ȵķе�֮��Ar�ĸߣ�������2��
��6����Ԫ������㺬�й¶Ե��ӣ��ܺ���Ԫ���γ���λ����������ȷ�Ĵ��Ǣۡ�
��7������ͭ�Ͱ�ˮ�������ֽⷴӦ������������ͭ����������ˮ����ʱ�����ڰ����ܺ�ͭ�����γ���λ�������Գ������ܽ⣬����������ӦʽΪCu2++2NH3?H2O= Cu(OH)2��+2NH4+��Cu(OH)2 +4NH3 =��Cu(NH3)4��2+ + 2OH-��

��ϰ��ϵ�д�
�����Ŀ





 ����
���� ���� �ֲ�ͬ�˶�״̬�ĵ��ӣ��� �ֲ�ͬ�ܼ��ĵ��ӡ�
���� �ֲ�ͬ�˶�״̬�ĵ��ӣ��� �ֲ�ͬ�ܼ��ĵ��ӡ� ���У��е��ɸߵ���
���У��е��ɸߵ��� �����д�������Ϊ��д��ѧʽ�� ��
�����д�������Ϊ��д��ѧʽ�� �� l3 B��[R(H2O)5Cl]Cl2��H2O
l3 B��[R(H2O)5Cl]Cl2��H2O [R(H2O)4Cl2]Cl��2H2O D��[R(H2O)3Cl3]��3H2O
[R(H2O)4Cl2]Cl��2H2O D��[R(H2O)3Cl3]��3H2O