��Ŀ����
��11�֣�Ԫ�����ڱ��У�һϡ������Ԫ��ԭ�ӵ��������ӹ���Ϊ4s24p6������ͬ���ڵ�A��B��C��D����Ԫ�أ����ǵ�ԭ����������������Ϊ2��2��1��7������A��C��Ԫ��ԭ�ӵĴ���������Ϊ8��B��D��Ԫ��ԭ�ӵĴ���������Ϊ18��E��D��Ԫ�ش���ͬ�壬���ڸ���Ԫ���У�E����̬�⻯��ķе���ߡ�
��1��BԪ�������ڱ��е�λ�� ; D������������ˮ����Ļ�ѧʽΪ______��
��2��E����̬�⻯����ͬ��Ԫ���зе���ߵ�ԭ���ǣ� ��
��3��A��C��Ԫ�ص�һ������ǰ�� ���ߣ�����ڡ���С�ڡ�����������A��C�ĵ��ʻ���A��C���⻯��Ĺ��壬������ˮ���ҷ�Ӧ�������壬���������ֹ���ֱ�������ˮ��Ӧʱ������n(���Ĺ���)��n(����)��n(ת�Ƶ���)=1:1:1��ϵ�ķ�Ӧ�Ļ�ѧ����ʽ_______________��
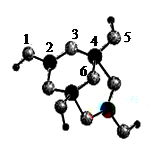
��4��BԪ�����γɶ���������B2+����NH3�γ�������[B��NH3��4]2+��������Ŀռ乹��Ϊ ����ͼ��ʾB��ij��Ԫ��X�γɵĻ����ᄃ��������û�������B��Xͨ�����Ӽ���ϣ��þ����������ӵ���λ��Ϊ ����B��Xͨ�����ۼ���ϣ���û�����Ļ�ѧʽΪ ��a ZnX b ZnX2 c ZnX3��
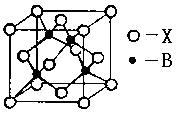
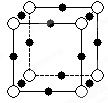
��5��A��E���γ����ӻ�����侧���ṹ��ͼʾ���û�����ĵ���ʽ_______��������A���Ӿ�������������E����Χ�ɵļ�������״��_______��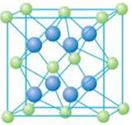
��1��BԪ�������ڱ��е�λ�� ; D������������ˮ����Ļ�ѧʽΪ______��
��2��E����̬�⻯����ͬ��Ԫ���зе���ߵ�ԭ���ǣ� ��
��3��A��C��Ԫ�ص�һ������ǰ�� ���ߣ�����ڡ���С�ڡ�����������A��C�ĵ��ʻ���A��C���⻯��Ĺ��壬������ˮ���ҷ�Ӧ�������壬���������ֹ���ֱ�������ˮ��Ӧʱ������n(���Ĺ���)��n(����)��n(ת�Ƶ���)=1:1:1��ϵ�ķ�Ӧ�Ļ�ѧ����ʽ_______________��
��4��BԪ�����γɶ���������B2+����NH3�γ�������[B��NH3��4]2+��������Ŀռ乹��Ϊ ����ͼ��ʾB��ij��Ԫ��X�γɵĻ����ᄃ��������û�������B��Xͨ�����Ӽ���ϣ��þ����������ӵ���λ��Ϊ ����B��Xͨ�����ۼ���ϣ���û�����Ļ�ѧʽΪ ��a ZnX b ZnX2 c ZnX3��

��5��A��E���γ����ӻ�����侧���ṹ��ͼʾ���û�����ĵ���ʽ_______��������A���Ӿ�������������E����Χ�ɵļ�������״��_______��

��1����4���ڵ�IIB�� HBrO4
��2��E����̬�⻯����Ӽ京�����,�ƻ�����Ҫ�ϸߵ������������۷е�ϸ�
��3�����ڡ�KH+H2O=KOH+H2����2�֣�
��4�������� 4 a ��5��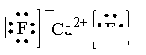 ������
������
��2��E����̬�⻯����Ӽ京�����,�ƻ�����Ҫ�ϸߵ������������۷е�ϸ�
��3�����ڡ�KH+H2O=KOH+H2����2�֣�
��4�������� 4 a ��5��
 ������
����������Ԫ�صĽṹ�������ʿ�֪��A��B��C��D��E��5��Ԫ�طֱ���Ca��Zn��K��Br��F��
��1��пλ��Ԫ�����ڱ��ĵ�4���ڵ�IIB�塣�������ǣ�7�ۣ�����������������ˮ����Ļ�ѧʽΪHBrO4��
��2��HF���Ӽ京��������ƻ�����Ҫ�ϸߵ������������۷е�ϸߡ�
��3��������Խǿ����һ������ԽС�����ԸƵĵ�һ�����ܴ��ڼصġ�����n(���Ĺ���)��n(����)��n(ת�Ƶ���)=1:1:1��˵����Ԫ�صĻ��ϼ��ǣ�1�ۣ�������KH������ʽΪKH+H2O=KOH+H2����
��4�������������νṹ��ͨ�����Ӽ���ϣ���ͭ�������ӡ����Ը��ݾ����ṹ��֪���þ����������ӵ���λ��Ϊ4����B��Xͨ�����ۼ���ϣ�����ݾ����ṹ��֪������̼ԭ����4����X��8��1/8��6��1/2��4�����Դ�ѡa��
��5��A��E�γ����ӻ�����CaF2�������ʽΪ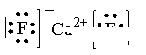 �������Ǹ����ӣ����Ը��ݾ����ṹ��֪��������������Ӿ�������������E���ӹ�����8����Χ�ɵļ�������״�������塣
�������Ǹ����ӣ����Ը��ݾ����ṹ��֪��������������Ӿ�������������E���ӹ�����8����Χ�ɵļ�������״�������塣
��1��пλ��Ԫ�����ڱ��ĵ�4���ڵ�IIB�塣�������ǣ�7�ۣ�����������������ˮ����Ļ�ѧʽΪHBrO4��
��2��HF���Ӽ京��������ƻ�����Ҫ�ϸߵ������������۷е�ϸߡ�
��3��������Խǿ����һ������ԽС�����ԸƵĵ�һ�����ܴ��ڼصġ�����n(���Ĺ���)��n(����)��n(ת�Ƶ���)=1:1:1��˵����Ԫ�صĻ��ϼ��ǣ�1�ۣ�������KH������ʽΪKH+H2O=KOH+H2����
��4�������������νṹ��ͨ�����Ӽ���ϣ���ͭ�������ӡ����Ը��ݾ����ṹ��֪���þ����������ӵ���λ��Ϊ4����B��Xͨ�����ۼ���ϣ�����ݾ����ṹ��֪������̼ԭ����4����X��8��1/8��6��1/2��4�����Դ�ѡa��
��5��A��E�γ����ӻ�����CaF2�������ʽΪ
 �������Ǹ����ӣ����Ը��ݾ����ṹ��֪��������������Ӿ�������������E���ӹ�����8����Χ�ɵļ�������״�������塣
�������Ǹ����ӣ����Ը��ݾ����ṹ��֪��������������Ӿ�������������E���ӹ�����8����Χ�ɵļ�������״�������塣
��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ

 ��L��M����Ԫ�ص�ԭ��������������X��Y������л���Ļ���Ԫ�أ�Z���������ӷֲ������P����ϵĵ��ӱ�S����ϵĵ��Ӷ�һ����L������Ԫ���е縺���ŵڶ�λ����ԭ�������������δ�ɶԵ��ӣ�M�����������������������Ӳ������δ�ɶԵ��ӡ��ش��������⣺
��L��M����Ԫ�ص�ԭ��������������X��Y������л���Ļ���Ԫ�أ�Z���������ӷֲ������P����ϵĵ��ӱ�S����ϵĵ��Ӷ�һ����L������Ԫ���е縺���ŵڶ�λ����ԭ�������������δ�ɶԵ��ӣ�M�����������������������Ӳ������δ�ɶԵ��ӡ��ش��������⣺